Abstract
Background:
Marginal zone lymphoma (MZL) accounts for approximately 10% of cases of non-Hodgkin's lymphoma (NHL). Rituximab in combination with chemotherapy has substantially improved treatment outcomes, but relapse is common. Although therapies for indolent NHL are FDA approved, no agents are specifically approved for the treatment of MZL. Ibrutinib, a first-in-class, once-daily inhibitor of Bruton's tyrosine kinase (BTK), is indicated by the US FDA for the treatment of patients (pts) with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), including CLL/SLL with del17p, mantle cell lymphoma with at least one prior therapy, and Waldenstrom's macroglobulinemia, and allows for treatment without chemotherapy. MZL is often linked to chronic infection, which can induce B-cell receptor signaling, resulting in aberrant B-cell growth and survival. By blocking BTK, a critical component of B-cell receptor signaling, ibrutinib may be an ideal therapy for the treatment of MZL. This study evaluated the efficacy and safety of single-agent ibrutinib in pts with relapsed/refractory (R/R) MZL.
Methods:
Pts had histologically confirmed MZL, ECOG PS of ≤2, life expectancy >3 months (mo) and had previously received ≥1 prior therapy including at least one CD20 monoclonal antibody (mAb)-containing regimen or monotherapy rituximab (RTX). All pts received ibrutinib 560 mg orally once daily until progression or unacceptable toxicity. The primary study endpoint was overall response rate (ORR) as assessed by an independent review committee (IRC) according to 2007 IWG criteria. ORR by investigator assessment is reported here, and ORR by IRC is forthcoming. Secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
Results:
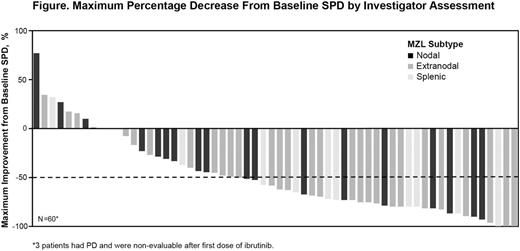
Sixty-three pts (extranodal [n=32], nodal [n=17], and splenic [n=14]) were enrolled. Median age was 66 years (range, 30-92); 92% had ECOG PS of 0-1. Median number of prior systemic therapies was 2 (range, 1-9) with 35% receiving ≥3 prior therapies. Four (6%) pts had a prior splenectomy, and 9 (14%) pts received prior radiotherapy. Seventeen pts (27%) had received only monotherapy RTX, and 40 (64%) had received at least one CD20 mAb-containing chemoimmunotherapy regimen; 33% of the pts had bone marrow involvement. The median time on study was 16.6 mo, and the median duration of therapy 11.7 mo. Per investigator assessment, ORR was 51% (6 CRs and 26 PRs). Thirty-eight percent of total pts had stable disease (SD), and the clinical benefit rate (CR+PR+SD) was 89%, with 87% having some tumor reduction (Figure). The overall median DOR was 19 mo (95% CI: 8.0-not reached [NR]). The median time to initial response was 4.2 mo, and the median PFS was 18 mo (95% CI: 12.2-NR). The most common adverse events (AEs ≥20%) of any grade included: fatigue (44%), diarrhea (43%), anemia (35%), nausea and thrombocytopenia (25% each), peripheral edema (24%), cough and arthralgia (22% each), dyspnea and URTI (21% each). Grade ≥3 AEs occurred in 40 pts (63%). The most common events were anemia (14%), pneumonia (8%), and fatigue (6%). Serious AEs of any grade occurred in 28 pts (44%), with grade 3/4 pneumonia being the most common AE (5 pts [8%]). Atrial fibrillation occurred in 4 pts (6%; all grade 1/2). Any-grade bleeding events occurred in 57% of pts, with 1 grade 3 event of hematemesis and 1 grade 5 cerebral hemorrhage. Three treatment-emergent AEs resulted in death due to disease progression, cerebral hemorrhage, and parainfluenza virus infection leading to multiple organ failure. Overall, 38 pts (60%) discontinued treatment (PD: 30%; AEs: 19%, pt decision: 5%; physician decision: 6%) and 40% continue treatment. The most common AE leading to treatment discontinuation was diarrhea in 2 pts (3%). At least 2 pts appeared to have experienced pseudoprogression at weeks 9 and 13, respectively, confirmed by further follow up.
Conclusions:
Single-agent ibrutinib achieved a high ORR and durable responses, and produced clinically meaningful tumor shrinkage yielding a high clinical benefit rate. Overall, the treatment was well tolerated. These results support BTK signaling blockade via single-agent ibrutinib as an effective, chemotherapy-free targeted strategy for R/R MZL.
Noy:Pharmacyclics, LLC, an AbbVie Company: Other: travel, accommodations, expenses, Research Funding. Thieblemont:Roche: Consultancy; Gilead: Consultancy; Janssen: Consultancy. Martin:Novartis: Consultancy; Celgene: Consultancy, Honoraria; Teva: Research Funding; Janssen: Consultancy, Honoraria, Other: travel, accommodations, expenses; Gilead: Consultancy, Other: travel, accommodations, expenses; Acerta: Consultancy. Flowers:Infinity: Research Funding; Pharmacyclics, LLC, an AbbVie Company: Research Funding; NIH: Research Funding; Millenium/Takeda: Research Funding; Acerta: Research Funding; Gilead: Consultancy, Research Funding; Mayo Clinic: Research Funding; ECOG: Research Funding; Roche: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; TG Therapeutics: Research Funding; AbbVie: Research Funding. Morschhauser:Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Janssen: Honoraria; Gilead Sciences: Consultancy, Honoraria. Collins:Takeda: Consultancy, Honoraria, Speakers Bureau. Ma:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Speakers Bureau; Novartis: Research Funding; Xeme: Research Funding; AbbVie: Research Funding. Coleman:Celgene: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics, LLC, an AbbVie Company: Research Funding, Speakers Bureau; Pfizer: Research Funding; Gilead: Consultancy, Research Funding, Speakers Bureau; GSK: Research Funding; Immunomedies: Equity Ownership, Other: Leadership; AbbVie: Equity Ownership; Karyoharm: Research Funding. Peles:Takeda: Consultancy, Honoraria, Other: travel, accommodations, expenses, Speakers Bureau; Novartis: Honoraria, Other: travel, accommodations, expenses, Speakers Bureau; Celgene: Honoraria, Other: travel, accommodations, expenses, Speakers Bureau; Onyx: Honoraria, Other: travel, accommodations, expenses, Speakers Bureau; SGN: Honoraria, Other: travel, accommodations, expenses, Speakers Bureau; Florida Cancer Specialists: Employment, Equity Ownership. Smith:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment, Other: travel, accommodations, expenses. Munneke:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment, Other: travel, accommodations, expenses. Dimery:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment, Other: travel, accommodations, expenses. Beaupre:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment, Other: travel, accommodations, expenses, Leadership, Patents & Royalties, Research Funding. Chen:Merck: Consultancy, Research Funding; Genentech: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Millenium: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal