Abstract
Background:
The genetic landscape of adult acute myeloid leukemias (AML) has been recently unravelled. This makes achievable the determination of a comprehensive profile of driver lesions for virtually all patients at diagnosis. Recent studies using multi-target minimal residual disease (MRD) strategies with around 1% sensitivity indicate that the clearance of all molecular events after chemotherapy is associated with better survival. To improve the clono-specificity and the sensitivity of this approach, after a precise determination of AML clonal composition, we combined cytogenetic, FISH, and high sensitivity deep sequencing technologies to monitor the MRD in a series of 69 patients.
Methods:
Forty-five consecutive patients reflecting the genetic diversity of AML were prospectively included and 24 patients were retrospectively studied. All patients received an anthracycline + cytarabine based regimen. The clonal architecture was established at diagnosis based on NGS-targeted resequencing (122 gene panel) and cytogenetic data. Lesions were next investigated in complete remission (CR). Based on the initial clonal composition, targeted resequencing panels were designed to improve the sensitivity by the use of unique molecular barcodes (Haloplex High Sensitivity, or HS-NGS assay). Cytogenetic events were evaluated by FISH.
Results:
In the 69 patients, a median of 4 genetic or chromosomal events were identified per patient (range 0-10). One patient had no evaluable target allowing MRD evaluation in 68/69 patients.
To determine the threshold of detection of the HS NGS assay, we analyzed the frequency of mutant reads in multiple samples expected to be wild type for 31 given SNVs and 2 indels. A consensus threshold of detection was set at a variant allele frequency (VAF) of 0.2% for all lesions.
In CR samples, early initiating events frequently persisted after treatment, especially mutations in DNMT3A, TET2, ASXL1, EZH2, IDH1, TP53, SRSF2, and U2AF1. Mutations in FLT3, NRAS, KIT, NPM1, CEBPA, WT1, IDH2 and BCOR were the most frequently cleared events.
Seven patients did not reach CR after one course, and two had no available material after one course. In the 59 remaining patients, we tested whether the global response level of all targets was associated with prognosis. We used the median VAF of the first events of all clonal architectures to separate good responder from poor responders (i.e. VAF = 1.66%). At 2 years, there was a trend to lower leukemia free survival (LFS) probability in poor responders (31.7+/-9.9% vs 51.7+/-9.8%, p=0.08) with no translation in overall survival (OS).
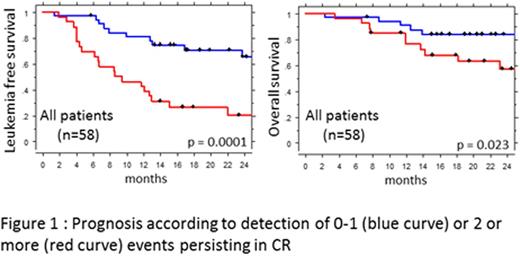
We next investigated if the persistence of two or more detectable markers was associated with prognosis. The 58 patients with more than one evaluable event were consequently separated in two groups: patients with 0 or 1 marker above the detection threshold after treatment (n=31), and patients with 2 or more detectable lesions (n=27). At 2 years, DFS was 64.9+/-9.3 % in patients with 0 or 1 detectable marker vs 19.8+/-8.7% in patients with 2 or more detectable markers (p=0.001). OS probability was higher in patients with 0 or 1 detectable marker 84+/-6.6% vs 57.1+/-10.5% (p=0.023). When focusing on the 40 patients with intermediate cytogenetics, persistence of 2 or more markers was associated with lower LFS (57+/-11.8% vs 19.4+/-10.5 p=0.0048) and with a trend to lower OS (85+/-8% vs 61+/-11.9% p=0.07). Similar results were observed when restricting the analyses to the 42 prospectively included patients (At 2 years: LFS 73+/-10% vs 24+/-10%, p=0.0026 and OS 90.2+/-6.6% vs 62.8+/-11.5%, p=0.036).
In 50 patients with 3 or more identified events, the persistence of 3 or more markers after one course was associated with a very high risk of relapse (DFS 23.5+/-10.3 % vs 75.8 +/-7.5% at one year, p<0.0001; median DFS at 7 months in the non-responder group and not reached after two years in the responder group), and lower OS probability (84.8+/-6.2 vs 45.2+/-13.5% at 2 years, p=0.026)
Conclusion
Our study shows the high prognostic value of a personalized multi-target clono-specific MRD evaluation that can be used in nearly all AML patients. Detection of two or more events in more than 0.4% cells after one course is associated with lower survival, in particular in intermediate cytogenetic patients. Larger studies are needed to confirm the results and to evaluate if this strategy could be useful to guide treatment decisions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal