Abstract
Introduction: African American (AA) race is associated with inferior survival in DLBCL compared to white (W) race (Shenoy, Cancer, 2011). This has been attributed to a disparity in administration of chemo-immunotherapy (Flowers, Cancer Epidemiol Biomarkers Prev 2012). Prevalence of human immunodeficiency virus (HIV) in AA with DLBCL is 8-fold higher than in W with DLBCL (Centers for Disease Control, 2014). We hypothesized that HIV infection is associated with a reduction in both administration of chemo-immunotherapy (rituximab) and overall survival (OS) in patients with DLBLC. We examined the relationship between race, HIV status, treatment with rituximab, and overall survival (OS) in a large cohort of United States Veterans with DLBCL with equal insurance access..
Methods We identified 3,227 DLBCL pts between 1998 and 2008 in the Veterans Health Administration Central Cancer Registry. We excluded pts with primary cutaneous or central nervous system DLBCL and pts treated with unknown agents outside the VHA, 2,163 met this inclusion criterion. Given Rituximab did not become standard of care in aggressive lymphomas until approximately 2002; we did a planned sub-analysis of the 1,672 patients diagnosed and treated after January 1, 2002. Data on stage, lactate dehydrogenase (LDH), medical co-morbidities (Charlson Comorbidity Index), date of death, treatment and supportive care medications were obtained for each patient. OS was defined as time from DLBCL diagnosis to date of death. Univariate comparisons were performed to identify differences in treatment and other prognostic variables between race groups, where appropriate. Multivariable analyses were performed using logistic regression and Cox proportional hazards modeling, producing odds-ratios (OR) or hazard ratios (HR), respectively.
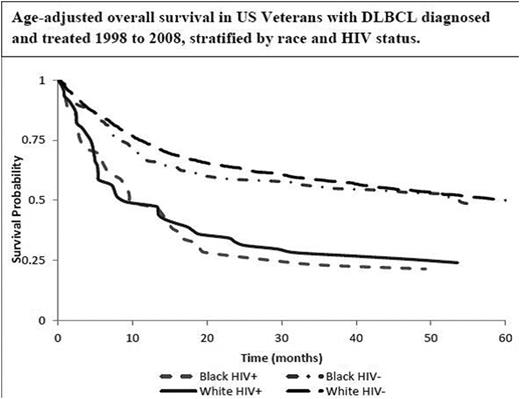
Results: Of the 2,163 pts that met inclusion criteria, 88% were W and 12% AA. There was a higher prevalence of HIV in AA patients versus W patients (21% v. 4%, p <0.0001). Consistent with previous reports, African American race was associated with a higher frequency of elevated LDH (64% vs. 54%, p = 0.003) and younger age at diagnosis (57.6 years vs. 66 years, p=0.0001). There was no significant difference between AA race and use of rituximab compared to W race (70.4% vs. 73.7%, P= 0.271). Patients who were HIV positive were significantly less likely to receive rituximab (OR = 0.1, 95% CI 0.06 - 0.18). Patients who were HIV positive had an increased risk of death (HR = 1.67, 95% CI 1.27 - 2.2) compared to those who were HIV negative. After adjusting for HIV status in a multivariable Cox analysis, there was no racial disparity in overall survival (HR = 1.02, 95% CI 0.83 - 1.25). Kaplan-Meier analysis of age-adjusted overall survival stratified by race and HIV status is presented in Figure 1.
Conclusions: Prior studies have shown detrimental survival outcomes in patient with HIV receiving Rituximab. Previously, it was also common practice to exclude Rituximab in treatment plans in HIV positive DLBCL patients (Kaplan, Blood, 2005).In this population of veterans with health insurance coverage, the observed racial disparity in survival after DLBCL diagnosis is largely explained by differences in the prevalence of HIV in the AA and W patient population. Thus, in order to reduce the racial outcome disparity among patients with DLBCL, public health efforts should focus on decreasing HIV transmission and enhancing HIV treatment in the AA community.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal