Abstract
Background: Allogeneic HCT is a potentially curative procedure for many hematologic malignancies, but is associated with several complications. Acute GvHD is a condition that affects 35-50% of allogeneic HCT recipients, typically occurs within 100 days after transplant, and can be life-threatening. Existing treatments are poorly tolerated and frequently ineffective. While the clinical consequences of acute GvHD are understood, the economic burden of the condition has not been well characterized.
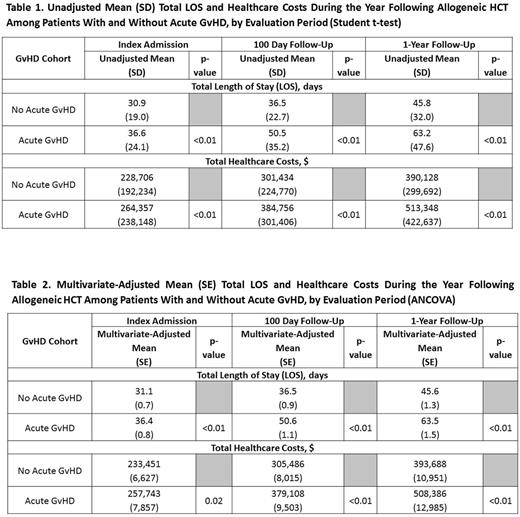
Methods: Using a large US healthcare claims database (Truven MarketScan® Commercial Claims and Encounters Database), patients aged ≥2 years old were identified who underwent allogeneic HCT between October 2009 and March 2013. Patients without continuous health plan enrollment or evidence of hematologic malignancy in the 6-month period prior to the "Index Admission" (hospital admission during which HCT was performed) were excluded. Patients were followed from the first day of the Index Admission until death, plan disenrollment, or one year; whichever occurred first ("Follow-Up"). Patients were classified into two cohorts: "Acute GvHD" based on ICD-9 CM diagnosis codes within first 100 days of Follow Up, or "No Acute GvHD". Total healthcare costs (inpatient care, outpatient care, outpatient pharmacy) and hospital length of stay (LOS) at discharge from Index Admission, the 100th day of Follow-Up, and the 365th day of Follow-Up were determined. Reimbursed amounts (plan payment plus patient liability) were used as a proxy for healthcare costs. Total healthcare costs and LOS between the two cohorts were compared for each of the three evaluation periods using Student's t-tests (unadjusted analyses), and analyses of covariance (ANCOVA; adjusting for differences in age, sex, plan type, geography, year of Index Admission, type of malignancy, Charlson Comorbidity Index score, and pre-admission healthcare cost).
Results: 1,635 patients underwent HCT and met all selection criteria (mean age was 48 years, 56% were men, acute myeloid leukemia was the most common malignancy); 42% met the criteria for inclusion in the Acute GvHD cohort. Univariate mean total healthcare costs for the Acute GvHD cohort (vs No Acute GvHD) were $36,651 greater during Index Admission, $83,322 greater at the 100th day of Follow-Up, and $123,220 greater at the end of the 1-year Follow-Up (all p<0.01); additional mean LOS (days) were 5.7 during Index Admission, 14.0 at the 100th day of Follow-Up, and 17.4 at the end of the 1-year Follow-Up (all p<0.01) [Table 1]. Multivariate-adjusted mean total healthcare costs were $24,292 greater during Index Admission (p=0.02), $73,622 greater at the 100th day of Follow-Up (p<0.01), and $114,698 greater at the end of the 1-year Follow-Up (p<0.01); additional LOS was 5.2 during the Index Admission, 14.1 at the end of the 100th day of Follow-Up, and 17.9 at the end of the 1-year Follow-Up (all p<0.01) [Table 2].
Conclusions: Over the one-year period following allogeneic HCT, patients who develop Acute GvHD experience over $100,000 more in total healthcare costs-and nearly three additional weeks in hospital-relative to those who do not. While healthcare claims appear to represent a good source with which to assess the impact of complications of HCT, confirmation with prospective clinical studies is recommended. Given that nearly one-half of patients develop Acute GvHD, our findings suggest that therapeutic strategies that prevent this complication may confer substantial savings to the healthcare system.
Grubb:Fate Therapeutics: Employment, Equity Ownership. Huse:Evidera: Employment; Fate Therapeutics: Consultancy. Alam:Evidera: Employment; Fate Therapeutics: Consultancy. Dychter:Fate Therapeutics: Employment, Equity Ownership. Wingard:Merck: Consultancy; Ansun: Consultancy; Fate Therapeutics: Consultancy; Gilead: Consultancy; Astellas: Consultancy. Berger:Evidera: Employment; Fate Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal