Abstract
Introduction: Anticoagulation is effective for the treatment of venous thromboembolism (VTE) in cancer patients, but it is also associated with an increased risk of bleeding. Previous clinical trials (e.g., CLOT and CATCH) of LMWH and warfarin for the treatment of VTE in cancer patients reported major bleeding in 3% to 6% of treated patients. The objective of this observational study was to compare the risk of major bleeding in cancer patients treated with anticoagulants for VTE in a real world setting.
Methods: Medical and pharmacy claims from the Humana Database from 1/1/2013 to 05/31/2015 were analyzed. Newly diagnosed cancer patients with a first VTE diagnosis occurring after their first cancer diagnosis, and with ≥1 dispensing of an anticoagulant within 7 days after their VTE diagnosis, were selected. Based on the first anticoagulant received, patients were classified into one of the following cohorts: LMWH, warfarin, and rivaroxaban (other agents not included due to low utilization). Inverse probability of treatment weights based on propensity score were used to adjust for differences between treatment cohorts for the following comparisons: LMWH vs. rivaroxaban, LMWH vs. warfarin, and rivaroxaban vs. warfarin. Patients were followed up until the earliest event, either treatment non-persistence (gap > 60 days between the end of the days of supply of a dispensing and the start date of the next dispensing), or end of data availability. Major bleeding events were identified using validated criteria (Cunningham et al., 2011). Kaplan-Meier rates at 3 and 6 months and Cox proportional hazards models were used to compare the risk of bleeding between different treatment cohorts. To better understand the risk of major bleeding in cancer patients unrelated to anticoagulation, a cohort of patients with cancer who did not have VTE and did not receive an anticoagulant was added as a control cohort.
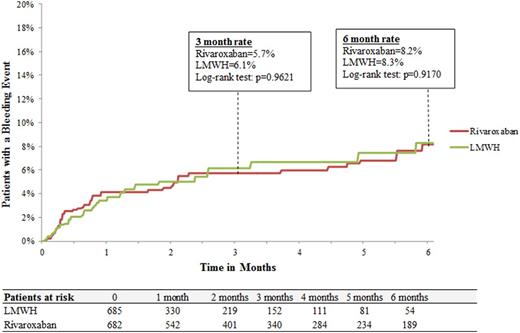
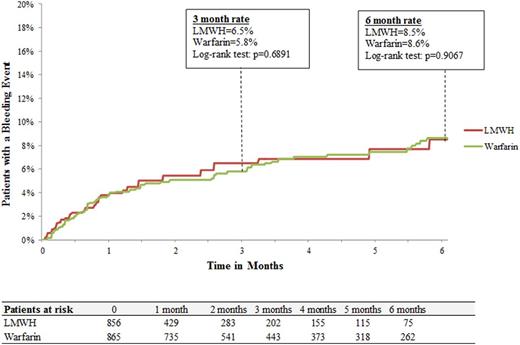
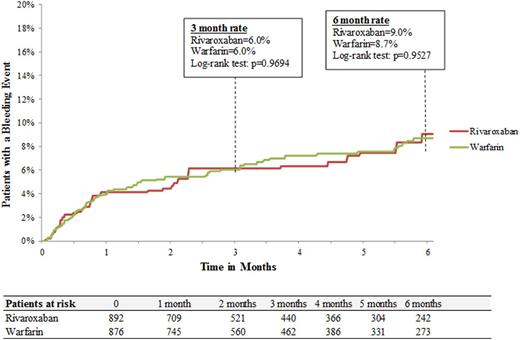
Results: A total of 2,428 patients (LMWH: n=660; warfarin: n=1,061; rivaroxaban: n=707) were included. Baseline demographic and clinical characteristics were well balanced among treatment cohorts. Median duration of therapy with LMWH was shorter than rivaroxaban (1.0 vs. 3.0 months, p<.0001) and warfarin (1.0 vs. 3.5 months, p<.0001). Rates of major bleeding for LMWH and rivaroxaban were 8.3% and 8.2%, respectively at 6 months with a hazard ratio (HRs [95% CI]) of 1.03 (0.64-1.65; Figure 1A). In the comparison between LMWH and warfarin cohorts, major bleeding rates were 8.5% and 8.6%, respectively at 6 months with hazard ratio (HRs [95% CI]) of 1.04 (0.69-1.57; Figure 1B). The risk of major bleeding was also similar for rivaroxaban and warfarin cohorts, 9.0% and 8.7%, respectively at 6 months with a hazard ratio (HR [95% CI]) of 1.01 (0.71-1.43; Figure 1C). For the control cohort of cancer patients without VTE and not receiving anticoagulation median follow-up was 5.6 months. Rates of major bleeding events for the control cohort were 2.6% and 4.2 % at 3 and 6 months, respectively.
Conclusion: This real world study of cancer patients treated for VTE found that the risk of major bleeding was similar for the 3 most widely prescribed anticoagulants in current clinical practice: LMWH, warfarin, and rivaroxaban. The observed rates of major bleeding were generally higher than what has been reported for LMWH and warfarin in the CLOT and CATCH trials. Patient characteristics such as older age (average age 73 years) could have contributed to the higher major bleeding rate seen in this study compared to the CLOT and CATCH trials, respectively.
Streiff:Portola: Research Funding; Janssen: Consultancy, Research Funding; Roche: Research Funding; CSL Behring: Consultancy, Research Funding. Milentijevic:Janssen Scientific Affairs: Employment, Equity Ownership. McCrae:Janssen: Membership on an entity's Board of Directors or advisory committees. Yannicelli:Janssen Scientific Affairs: Employment, Equity Ownership. Fortier:Janssen Pharmaceuticals: Research Funding. Nelson:Janssen Scientific Affairs: Employment, Equity Ownership. Laliberté:Janssen Scientific Affairs: Research Funding. Crivera:Janssen Scientific Affairs, LLC, Raritan, New Jersey: Employment, Equity Ownership. Lefebvre:Janssen Scientific Affairs: Research Funding. Schein:Johnson & Johnson: Employment, Equity Ownership, Other: Own in excess of $10,000 of J&J stock. Khorana:Roche: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Halozyme: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Leo: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Janssen Scientific Affairs, LLC: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal