Abstract
The recent number of US Food and Drug Administration (FDA) drug approvals for the treatment of multiple myeloma (MM) is unprecedented.Four new therapeutic agents,panobinostat,daratumumab,elotuzumab, andixazomibwere approved in 2015 matching the record of seven new-agents and 16 regulatory approvals during the past 12 years. New therapies have dramatically improved life expectancy for patients with MM. The therapeutic benefit of newer agents, combined with the incurable and relapse-remission nature of disease, has further propagated the interest of pharmaceuticaland academic investigations. Thus, a multitude of anti-myeloma compounds have been proposed, evaluated in pre-clinical studies and tested in phase I trials. A major hurdle to recruiting patients onto phase I oncology trials has the assumption that these trials offer low therapeutic benefit, which makes phase I trial recruitment a last resort(Simon et al. JCO, 2004).The status of MM phase I trials in the past 12 years has not been reviewed in detail. The aim of this study is to determine overall therapeutic benefit and risks forptsrecruited in phase I trials from 2004 to 2015 and to analyze the role of potential factors affecting outcomes.
Methods: Phase I trials for MM conducted from 2004-2015 were identified from searches of MEDLINE, Cochrane Library and scientific meetings (Fig. 1). Data was extracted by two independent reviewers based upon the same algorithm and significant overlap in assignment to assess inter-observer heterogeneity.
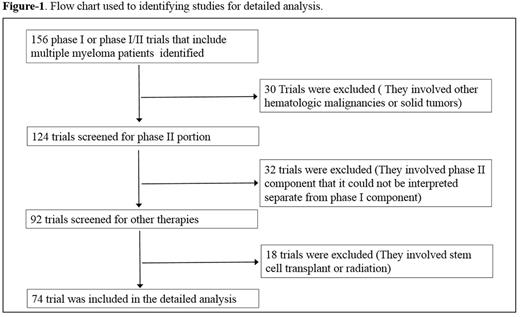
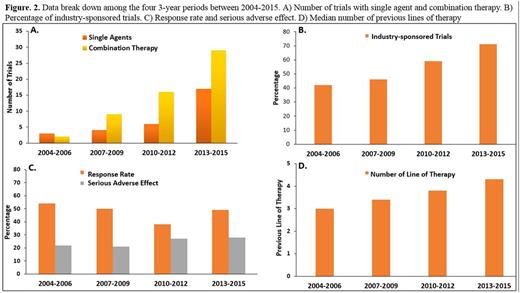
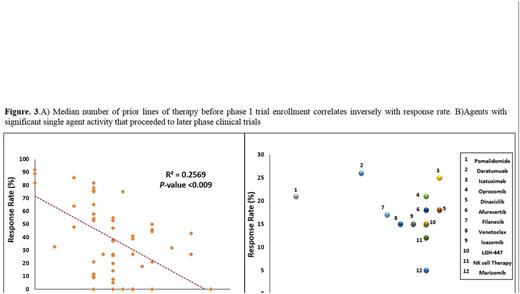
Results: Trials (n=74) were selected and included a total of 2408 enrolled pts (Fig. 1). The median number of pts/trial was 29 (range: 5-84), 56% of pts male, 44% female, median age was 67 years (range: 55-71) with increasing trend toward the end of the study period. The performance status was 2 or better in all trials. 39% of the trials evaluated a single agent (excluding corticosteroids) and the remainder evaluated combination therapies (Fig. 2A). 12 (42%) trials tested small molecules and 16 (68%) used monoclonal antibodies as single agent. 90% of the trials were conducted based on 3+3 design. The proportion of industry-sponsored trials increased progressively in the study period (Fig. 2B). The ratio of the initial dose to the Maximum Tolerated Dose (MTD) was 0.29 indicating significant portion of enrolled pts were potentially undertreated, most likely due to dominance of 3+3 design. 1107 of the 2408 pts responded to the study drugs which resulted in overall response rate (ORR) of 42% (range: 0-91). Median ORR was significantly different in trials with single agent vs. combination therapy (20% vs. 40%, respectively, p-value<0.01). The Median number of prior treatment lines showed an increasing trend toward the end of the study period (Fig. 2D) that correlated inversely with response rate (Fig. 3A). The effect of number of prior lines of therapy on response rate remained significant after multivariate analysis taking age, the year of publication and ratio of initial dose to the MTD into account. There were 7 therapy-related in all studies (overall death rate: 0.4 %). Pts who participated in combination therapy phase I clinical trials had more SAEs than single agents (29% vs 16%, HR: 1.35, p-value: 0.04). Median serious adverse events (SAE) was 22% (range: 0-44) (Fig. 2C). Response rates and SAE rate were not statistically difference between the 4 periods of the study (2004-06, 2007-09, 2010-12 and 2013-15, p-value=0.3).Daratumuab,ixazomib,pomalidomide,Isatuximab,marizomib,oprozomib,filanesib,dinaciclib,venetoclax and LGH-447, had single agent anti-myeloma activity and proceeded to later phase clinical trials (Fig. 3B).
Conclusions: Our analysis indicates that the therapeutic benefit for patients recruited onto MM phase I trials was significantly higher than that reported for phase I trial of all cancer types (Horstmann et al. NEJM. 2005).Our results suggest an inverse correlation between the number of prior lines of therapies and the response rate that support earlier patient entry onto phase I studies to increase the therapeutic benefit.Also, our analysis shows that despite an increase in the number of compounds tested in MM phase I trials during the past 12 years, the overall toxicity from these trials has not increased. It is also possible that less patients would be undertreated by utilizing phase I designs other than 3+3 that deliver therapeutic dosage to a larger portion of enrolled patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal