Abstract
Background: Primary mediastinal B-cell lymphoma (PMBL) is a distinct diffuse large B-cell lymphoma that occurs in adolescents and young adults. Retrospective studies suggest dose-intensive therapy is more effective than R-CHOP, and support a role for mediastinal radiation following R-CHOP. We demonstrated that DA-EPOCH-R is effective and obviates the need for mediastinal radiation, thus eliminating the risk of long-term toxicities (NEJM 2013;368:1408-16). End of therapy (EOT) FDG-PET is often performed to assess residual mass activity. The clinical value of EOT negative FDG-PET is under investigation in an international study in which FDG-PET negative patients are randomized to receive radiation or observation following immunochemotherapy (IELSG 37). All patients with an EOT positive FDG-PET receive mediastinal radiation. Herein, we investigate the predictive value of EOT FDG-PET following DA-EPOCH-R and provide updated results from the National Cancer Institute (NCI) and Stanford University Hospital publication of PMBL (NEJM 2013;368:1408-16).
Methods: Ninety-two patients with newly diagnosed PMBL were treated with DA-EPOCH-R (Table 1). DA-EPOCH-R was administered for 6-8 cycles as previously described and no patients received up-front radiation. EOT FDG-PET was performed in 76 (83%) patients and scored retrospectively according to Deauville criteria with scores 4 to 5 called positive and scores 1 to 3 called negative. Patients with EOT positive FDG-PET scans were followed with serial scans to distinguish persistent disease from resolving inflammatory changes. The NCI and Stanford cohorts showed no significant differences in outcome and were therefore combined for analysis of event free survival (EFS) and overall survival (OS). Analyses of EFS and OS in patients with Deauville positive and negative EOT FDG-PET scans were also performed.
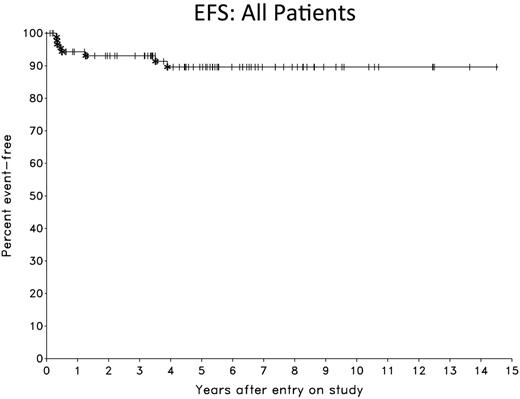
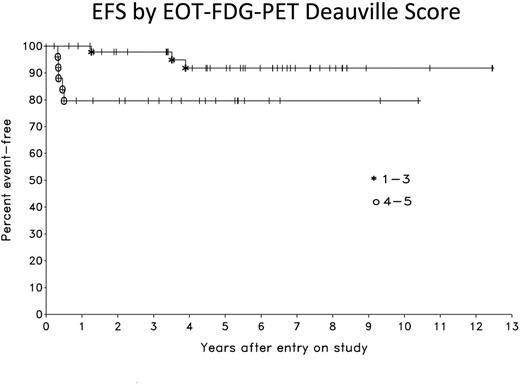
Results: Patient characteristics were similar in the NCI and Stanford cohorts (Table 1). Overall, the median age was 31 years (range, 18 to 68), 59% were female, 59% had mediastinal masses > or = 10 cm and 20% of patients had stage IV disease. At a median follow-up of 5.3 years, the EFS and OS are 90% and 94%, respectively (Figure 1). Of 76 EOT FDG-PET scans performed, 25 (33%) were positive (Deauville score 4 or 5) and 51 (67%) were negative (Deauville score 1 to 3). Only 1 (2%) patient with an EOT negative FDG-PET scan relapsed (at 1 year), and 5 (20%) patients with an EOT positive FDG-PET scan had residual disease and received further therapy. EOT FDG-PET had a sensitivity and specificity of 83% and 71%, respectively, and a positive and negative predictive value of 20% and 98%. Comparison of outcome for EOT FDG-PET negative and positive patients at 5.3 years was 92% and 80% for EFS (p= 0.043), respectively, and was 94% and 91% for OS (p= 0.37) (Figure 2). Serial monitoring of false positive EOT FDG-PET scans showed reduction or occasionally stabilization of SUV, which is consistent with inflammatory causation. There were no events in the 16 patients without evaluable EOT FDG-PET scans resulting in an EFS of 100%. This EFS, however, was not significantly different than that of patients with evaluable EOT FDG-PET scans.
Conclusions: DA-EPOCH-R (without radiation) is highly effective in PMBL and has an EFS of 90% with long-term follow-up. An EOT negative FDG-PET has a negative predictive value of 98%. Even among patients with an EOT positive FDG-PET, 80% are cured of disease and achieve long-term remission. These results demonstrate that EOT Deauville 4-5 FDG-PET scans following DA-EPOCH-R do not accurately identify patients with residual disease in most cases and should not be used to justify mediastinal radiation. In the absence of disease progression on CT scans, patients with EOT positive FDG-PET scans should be carefully monitored with short interval (e.g. 4-6 weeks) repeat FDG-PET scans to assess progressive SUV changes and need for biopsy before instituting further therapy.
Advani:Kyowa Hakko Kirin: Consultancy, Honoraria; Infinity: Research Funding; FortySeven: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Research Funding; Sutro: Consultancy, Honoraria; Spectrum: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Juno: Consultancy, Honoraria; Stanford University: Employment; Millennium: Research Funding; Kura: Research Funding; Celgene: Research Funding; Regeneron: Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; Janssen: Research Funding; Seattle Genetics: Research Funding; Agensys: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal