Abstract
Background
There is no standard treatment for R/R MCL that fail first line treatment. Non cross resistant regimens are usually used, which provided sometimes good overall response rate (until 93%) but with a minor disease control (PFS<2years). [1] The main objective of these salvage regimens is to bypass disease resistance, to obtain more profound ( deep or durable) response and to ensure, in younger patients, the option of performing autologous or allogenic stem cell transplantation. For older patients prolonging disease free survival is the aim. The new combination RiVBD (Rituximab-Bendamustine-Bortezomib-Dexametasone) has recently shown to be an effective regimen in frontline for eldery patients with a good tolerability profile (NCT 01457144). [2] Many French centers have also used this association for the R/R patients.
Aim
To explore the efficacy of the RiBVD regimen in the salvage therapy setting following failure of one, two or more prior treatments.
Methods
We proposed to all French LYSA partner centers a survey to retrospectively evaluate the efficacy of the RiBVD regimen in R/R MCL patients, regardless of prior treatments used. The RiBVD regimen comprises : Rituximab 375mg/sqm D1, Bendamustine 90mg/sqm D1 and D2, bortezomib 1,3mg/sqm D1, D4, D8, D11 and dexamethasone 40 mg D2. Analysis was performed in June 2016.
Results
From January 2012 to December 2015, 49 patients from 17 French hematological centers were recruited to the study. The median age was 72 years (50-91y) with 14 young (<65y) and 35 older patients (> 65y). Thirty eight cases presented with classic MCL variant and 11 had a blastoid variant. All patients but one were CD20+, CD5+, CD10- and were positive CYCLIN D1 expression and/or the t(11;14)(q13;q32). Eighteen patients presented a t(11;14) (q13;q32).The CYCLIN D1 negative patient had a t(11;14).
Treatment history: Twenty seven patients received RiBVD in second line, 12 in third line and 10 patients after the third lines. Twenty two patients were refractory to their previous line and 27 were in relapse. Before RiBVD 44/49 patients (90%) had received high dose cytarabine, 3 Ibrutinib and 14 patients were intensified (11 at diagnosis, 3 in relapse).
Efficacy: The global overall response rate (ORR) was 75% (37/49, 23 CR and 14 PR). For patients treated in 2nd line, the ORR was 85% (23/27, 16 CR and 7 PR), in 3nd line 58% (7/12, 4 CR and 3 PR), and 70% (7/10) for the others (3 CR and 4 PR). Young patients had an ORR of 64% (9/14, 8 CR, 2 RP) and elderly pts 77% (27/35, 15 CR, 12 PR). For relapsed and refractory pts the ORR was respectively 85% (23/27, 15 CR and 8 PR) and 63% (14/22 with 8 CR and 6 PR). For Classic and blastoid variants the ORR was 81.5% (31/38, 20 CR and 11 PR) and 54% (6/11, 3 CR and 3 PR) respectively. Note that 2/3 pts receiving RiBVD regimen post Ibrutinib failure, reached PR (n=2) and showed stable disease (n=1).
Major toxicities were seen in 31 pts (63%) with grade 3/4 hematological toxicity in 22 pts, grade 3 neurotoxicity in 3 pts, grade 3/4 cardiotoxicity in 3 pts, grade 3/4 infectious complications in 8 pts, grade 4 fatigue in 3 pts and grade 3 digestive-tract or cutaneous toxicity in one pt each.
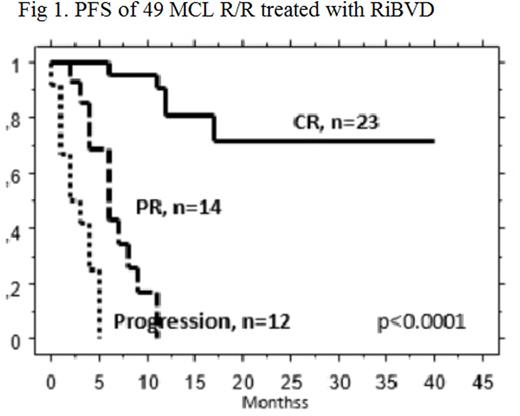
At the update point, 17 pts had died, 15 for lymphoma progression, 2 for TRM while experiencing a CR (infectious and leukemia). The follow-up of the 32 surviving pts was 14.5 month. The median PFS was 9 months for the 49 pts. The PFS was statistically affected by the pathologic type (classic vs Blastoid, p=0.03), the number of prior treatment (one vs >one, p=0.04) and response to RiBVD (CR vs PR vs no response, p<0.0001 with a median PFS not reached for CR pts, 6 months for PR and 2 months for no response. The age (<65 vs >65) or the state (relapse or refractory) at the time of RiBVD had no impact on PFS.
Conclusion
The RiBVD regimen which shows remarkable efficacy in frontline treatment of elderly MCL pts, shows potential as a salvage therapy for refractory or relapsed MCL following cytarabine based treatment. This is particularly true for the 47% of patients achieving CR for which 2 years PFS was 71% regardless of their age.
1. Cheah CY, Seymour JF, Wang ML. Mantle Cell Lymphoma. J Clin Oncol 2016; 34: 1256-1269.
2. Gressin R, Callanan M, Daguindau N et al. Frontline therapy with the RiBVD regimen elicits high Clinical and Molecular Response Rates and long PFS in elderly patients Mantle Cell Lymphoma (MCL); Final Results of a Prospective Phase II trial by the LYSA group. Blood 2014.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal