Abstract
Background: Patients with classical Hodgkin lymphoma (cHL) who progress after brentuximab vedotin (BV) have a poor prognosis. cHL frequently harbors genetic alterations at the 9p24.1 locus, resulting in the overexpression of the PD-L1 and PD-L2 immune checkpoint ligands. Pembrolizumab is a humanized monoclonal antibody that blocks the interaction between the PD-1 receptor and PD-L1/PD-L2, and can restore antitumor immune activity in several different tumors. Based on its likely genetically driven dependence on PD-1, cHL was included as an independent expansion cohort in the KEYNOTE-013 study (NCT01953692), a multicenter, multicohort phase 1b trial of pembrolizumab in patients with hematologic malignancies. Updated results from this cohort, including long-term efficacy, are presented.
Methods: Key eligibility criteria for the cHL cohort of KEYNOTE-013 included relapse after or ineligibility for autologous stem cell transplantation (ASCT), and relapse after or refractory to BV treatment. Pembrolizumab was administered intravenously at a dose of 10 mg/kg every 2 weeks for up to 2 years or until confirmed progression or unacceptable toxicity. Response was assessed at week 12 and every 8 weeks thereafter according to the International Harmonization Project 2007 criteria. The primary end points were safety and complete remission (CR) rate (CRR); secondary end points included overall response rate (ORR) and duration of response (DOR). Patients who achieved a CR could opt to stop treatment after 24 weeks provided that they received at least 2 doses after CR. This report includes CRR, ORR, and DOR by blinded independent central review (BICR).
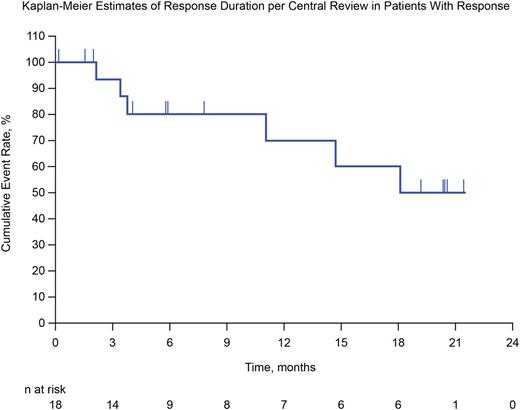
Results: At the time of data cutoff on June 3, 2016, 31 patients were enrolled, and all were evaluable for analysis. Median follow-up duration was 24.9 months (range, 7.0-29.7 months). The median number of prior lines of therapy was 5 (range, 2-15), 74% of patients had failed prior ASCT, and by design 100% had failed prior BV. Per investigator review, ORR was 65%, and CRR was 19%. Per BICR, ORR was 58% (18/31), with 6 patients (19%) achieving CR and 12 (39%) partial remission; 7 patients (23%) had stable disease as their best response. Median DOR was not reached, with a range of 0.0+ to 21.4+ months (95% CI, 3.7 months to not reached) (Figure).
An analysis with hierarchical mutually exclusive categories of refractory disease (RD; defined as no response to ≥1 prior line of therapy) or relapse after ≥3 prior lines of therapy (Re ≥3) was conducted. Per BICR, the ORR was 56% in RD (n = 27 patients) and 75% in Re ≥3 (n = 4). As of the data cutoff date, 3 patients (10%) remained on treatment, 5 (16%) completed 2 years of treatment, and 23 (74%) discontinued treatment: 3 (10%) for toxicity, 14 (45%) for progressive disease, 3 (10%) per physician decision (all ultimately underwent allogeneic SCT), 1 in CR (underwent allogeneic SCT), 1 for clinical progression, and 1 who withdrew consent. Per BICR, median progression-free survival (PFS) was 11.4 months; 6-month and 12-month PFS rates were 66% and 48%, respectively. Median overall survival (OS) was not reached; 6-month and 12-month OS rates were 100% and 87%, respectively.
Conclusions: With nearly 2.5 years of median follow-up, the present results demonstrate that a subset of heavily pretreated patients who failed BV therapy can achieve a long-term response with single-agent pembrolizumab, without consolidative therapy. PD-1 blockade may offer a new treatment paradigm for patients with relapsed/refractory cHL, supporting the hypothesis that this tumor has a genetic dependence on the PD-1 pathway.
Armand:BMS: Consultancy, Research Funding; Sequenta: Research Funding; Roche: Research Funding; Infinity: Consultancy; Merck & Co., Inc.: Consultancy, Research Funding; Sigma Tau: Research Funding; Tensha: Research Funding; Otsuka: Research Funding. Shipp:Cell Signaling: Honoraria; Bayer: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Merck, Gilead, Takeda: Other: Scientific Advisory Board. Ribrag:Incyte: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Nanostring: Membership on an entity's Board of Directors or advisory committees; ArgenX: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Infinity: Membership on an entity's Board of Directors or advisory committees; Pharmamar: Membership on an entity's Board of Directors or advisory committees; Esai: Membership on an entity's Board of Directors or advisory committees. Michot:Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Zinzani:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Infinity: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Sandoz: Membership on an entity's Board of Directors or advisory committees; Millennium: Membership on an entity's Board of Directors or advisory committees. Kuruvilla:Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Honoraria; Celgene: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Merck: Honoraria; Roche Canada: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Lundbeck: Honoraria. Zhu:Merck: Employment. Ricart:Merck & Co.: Employment; Pfizer: Equity Ownership. Balakumaran:Merck & Co.: Employment, Other: stock, stock options. Moskowitz:Pharmacyclics: Research Funding; Merck: Consultancy, Research Funding; Genentech: Consultancy; Seattle Genetics: Consultancy, Research Funding; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal