Abstract
BACKGROUND: Patients (pts) with follicular lymphoma (FL) who have progression of disease within 2 years of immunochemotherapy have poor outcomes and represent a distinct group for whom development of new therapies is warranted (Casulo et al. J Clin Oncol 2015). Autologous T cells genetically modified to express a chimeric antigen receptor consisting of an external anti-CD19 single chain murine antibody domain with CD3ζ and 4-1BB signaling domains (CTL019 cells) can mediate potent anti-tumor effects in pts with relapsed or refractory chronic lymphocytic leukemia, acute lymphoblastic leukemia, and B cell lymphomas. We evaluated the safety and efficacy of CTL019 cells in pts with relapsed or refractory FL as part of an ongoing phase IIa clinical trial (NCT02030834).
METHODS: Eligible pts have CD19+ FL with progression of lymphoma <2 years after second or higher line of therapy (not including single agent monoclonal antibody therapy), no available curative treatment options, a limited prognosis (<2 years anticipated survival), and responsive or stable disease with most recent therapy. After steady state apheresis to collect peripheral blood leukocytes, pts receive lymphodepleting chemotherapy based on disease burden, clinical status, and past therapies. One to 4 days after chemotherapy, pts receive a single dose of CTL019 cells by intravenous infusion. Following the CTL019 infusion, patients receive no further therapy. Peripheral blood and marrow samples are collected for immunophenotypic, cytokine, and molecular studies at pre-specified times after T cell infusion. Initial tumor response assessment is performed 3 months (mo) after T cell infusion using International Working Group response criteria. Time to next treatment (TTNT) is measured from the start of previous line of therapy to date of T cell infusion and date of T cell infusion to the time of next treatment for lymphoma. Enrollment started in February 2014; data are reported through 24 July 2016.
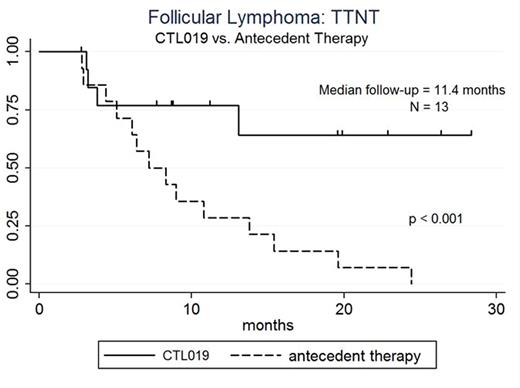
RESULTS: To date, 14 pts with FL are enrolled (9 pts with FL grade 1-2 and 5 pts with FL grade 3a). The median age is 59 years (range: 43-72), male: female ratio is 7:7, median number of prior therapies is 5 (range: 2-10), and number of pts with prior transplant is 3 (21%; 2 auto, 1 allo transplant). Ann Arbor stages at enrollment are: Stage IV 9 pts (64%), Stage III 3 pts (21%), and Stage II 2 pts (14%); 4 pts (29%) have bone marrow involvement. LDH is increased in 9 pts (64%). ECOG PS is 0 in 10 pts (71%) and 1 in 4 pts (28%).FLIPI score at enrollment is high risk in 10 (71%) pts, intermediate risk in 2 (14%), and low risk in 2 (14%). All pts had previously received rituximab, either alone or in combination, with failure to respond or progression of disease after rituximab-based combination therapy. As of July 24, 2016, 14 patients received the protocol-specified dose of CTL019 cells.Lymphodepleting chemotherapy regimens are bendamustine (6 pts), cyclophosphamide (2 pts), cyclophosphamide-fludarabine (1 pt), radiation-cyclophosphamide (3 pts), EPOCH (1 pt), and carboplatin-gemcitabine (1 pt). Median CTL019 cell dose/kg is 6.08E+06 (range: 3.08-8.87E+06). Median peak CTL019 cell expansion in blood occurred 7 days after infusion (range: 7-14 days); there is no difference in peak expansion between responders and non-responders.Fourteen pts are evaluable for response, and 13 pts are evaluable for response duration. Overall response rate (ORR) at 3 mo is 79% (11/14 pts) with complete response (CR) rate 50% (7/14 pts). At 6 mo response assessment, 2 of 3 pts with partial response (PR) converted to CR. Best response is CR in 9 of 14 pts (64%). One pt with PR at 3 mo, who remained in PR at 6 and 9 mo, ultimately had progression of disease at 13 mo. At the median follow-up 11.4 mo from CTL019 infusion, the proportion of pts progression-free is 77% (95%CI: 45-92%) and the median TTNT not yet reached compared to median TTNT 7.2 mo (95%CI: 4.4 to 13.8 mo) for antecedent therapy (p < 0.001, log rank, see figure). Cytokine release syndrome (CRS) occurred in 6 pts (4 grade 2; 1 grade 3, 1 grade 4) and did not predict response. Other toxicities included one episode of grade 5 encephalitis, possibly related to therapy; this pt remained in CR at the time of death.
CONCLUSIONS: These results suggest that a single treatment with CTL019 cells with no subsequent therapy has efficacy in FL and can improve time to next treatment compared with prior therapy in patients with poor prognosis, relapsed or refractory FL.
Svoboda:Celgene: Research Funding; Seattle Genetics: Research Funding; Pharmacyclics: Research Funding. Nasta:Millennium Pharmaceuticals: Research Funding. Porter:Immunovative Therapies: Other: Travel, Accommodations, Expenses; University of Pennsylvania: Patents & Royalties; Novartis: Research Funding; Novartis: Consultancy; Incyte: Honoraria; Genentech/Roche: Equity Ownership; Genentech/Roche: Employment. Mato:Pharmacyclics: Consultancy; TG Therapeutics: Consultancy; Abbvie: Research Funding; Acerta Pharma: Research Funding; Gilead Sciences: Research Funding; ProNAi: Research Funding; TG Therapeutics: Research Funding; Theradex: Research Funding; Gilead Sciences: Consultancy; Abbvie: Consultancy. Lacey:Novartis: Research Funding. Melenhorst:Novartis: Research Funding. Chew:Novartis: Research Funding. Hasskarl:Novartis: Employment. Marcucci:Novartis: Research Funding. Levine:Novartis: Patents & Royalties, Research Funding; GE Healthcare Bio-Sciences: Consultancy. June:Novartis: Honoraria, Patents & Royalties, Research Funding; Novartis: Honoraria, Patents & Royalties, Research Funding; Celldex: Consultancy, Equity Ownership; Tmunity Therapeutics: Equity Ownership; Johnson & Johnson: Honoraria; Pfizer: Honoraria; Immune Design: Consultancy, Equity Ownership. Schuster:Gilead: Research Funding; Nordic Nanovector: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Research Funding; Hoffman-LaRoche: Research Funding; Janssen Research & Development: Research Funding; Genentech: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal