Abstract
Background: Molecular characterization of tumors currently relies in large part on invasive tissue biopsies, which can often be unavailable or limiting. For example, while biological heterogeneity between diffuse large B-cell lymphoma (DLBCL) patients can be stratified using classification of cell-of-origin (COO), current methods relying on tumor gene expression profiles (GEP) or multi-parametric immunohistochemistry (IHC) require adequate tissue, and can be hampered by modest accuracy and reproducibility. Circulating tumor DNA (ctDNA) is an emerging noninvasive biomarker for tumor detection with potential for DLBCL disease monitoring. Here, we evaluated the utility ofctDNA for COO classification of patients with DLBCL.
Methods: We previously built and trained a novel DLBCL COO Naive Bayesian classifier, using somatic alterations in 32 genes and associated with COO subtypes that were previously classified by Affymetrix mRNA GEP of frozen tumors (n=142 Training; Scherer et al., ASH Annual Meeting 2015 / ASCO Annual Meeting 2016). Here, we applied CAPP-Seq, a capture-based targeted high-throughput sequencing (HTS) method (Newman et al., Nat Med 2014), to genetically profile and classify 146 DLBCL patients in independent cohorts. We employed a test/validation framework (n=76 Test, n=70 Validation) to classify patients diagnosed and treated at Stanford University, MD Anderson Cancer Center, or at the University of Eastern Piedmont, Italy. We compared COO classification from pre-treatment plasma samples (n=119), tumor biopsy tissues (n=82, of which 71% were from FFPE), or both (n=41) against the current clinical standard immunohistochemistry method (Hans algorithm, n=117). Kaplan Meier analyses and Cox proportional-hazard models were used to assess clinical utility of biopsy-free and tumor genotyping for prediction of clinical outcomes from time of DLBCL diagnosis.
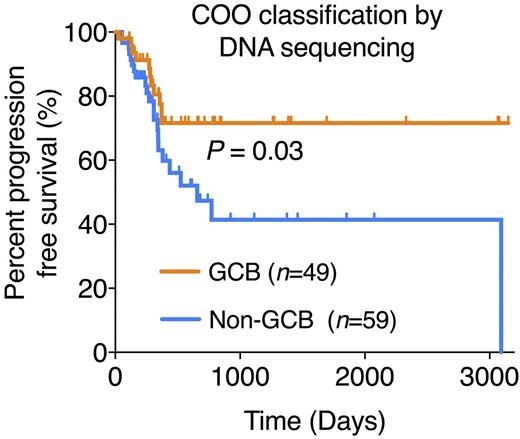
Results: Genotyping of both test and validation cohorts using either tumor or plasma samples allowed COO classification in 100% of patients (GCB=77 / non-GCB=69) with 77% overall concordance to blinded and centralized Hans IHC classification. As expected, histologically transformed (tFL/DLBCL) and double-hit DLBCLs (MYC and BCL2/BCL6 translocated) were strongly biased toward GCB DNA subtype (26/29, 90%), while primary central nervous system lymphomas were almost invariably classified as ABC/non-GCB by ctDNA (6/7, 87%). The concordance between DNA-based COO classification from tumor vs. plasma was 88% (36/41). In the test cohort, COO classification by genotyping from either tissue or plasma was significantly associated with PFS (P=0.003 and 0.02, respectively, Cox proportional-hazard), while classification by Hans criteria failed to show a significant survival difference (P=0.25). The superior outcome of patients with GCB DNA COO subtype was validated when extended to the full cohort (P=0.02, Cox proportional-hazard; P=0.03, log-rank (Figure 1)). We observed a similar, albeit not significant, trend for overall survival.
Conclusions: DLBCL COO subtypes can be accurately classified using somatic alterations detectable as circulating tumor DNA. We envision that this approach might help overcome some of the limitations of mRNA GEP and protein/IHC-based methods, including the requirement for invasive biopsies, tissue availability, and suboptimal assay performance. Furthermore, this strategy could support future clinical trials in helping to identify poor-risk groups at diagnosis, to guide future therapy selection, and to improve treatment decisions for patients with DLBCL.
Newman:Roche: Consultancy. Lovejoy:Roche: Employment. Levy:Kite Pharma: Consultancy; Five Prime Therapeutics: Consultancy; Innate Pharma: Consultancy; Beigene: Consultancy; Corvus: Consultancy; Dynavax: Research Funding; Pharmacyclics: Research Funding. Gaidano:Janssen: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Morphosys: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Speakers Bureau. Diehn:Roche: Consultancy; Novartis: Consultancy; Quanticel Pharmaceuticals: Consultancy; Varian Medical Systems: Research Funding. Alizadeh:Gilead: Consultancy; Celgene: Consultancy; Genentech: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal