Abstract
Background: Crenolanib is a type I oral FLT3 TKI, which inhibits both FLT3-ITD and FLT3-TKD mutations (D835, N841, etc). Crenolanib has a half-life of 6-8 hours and does not accumulate with repeated dosing. Crenolanib does not inhibit c-kit at clinically achievable concentrations, potentially allowing for full count recovery even when combined with myelosuppressive chemotherapy. We report here the first analysis of a phase II trial evaluating the tolerability and efficacy of crenolanib combined with standard induction chemotherapy in patients (pts) with newly diagnosed FLT3 mutant AML.
Methods: Pts (≥ 18yrs) (no upper age limit) with newly diagnosed AML characterized by FLT3 (ITD and/or TKD) mutations were enrolled. Pts received standard 7+3 induction with cytarabine 100 mg/m2/d for 7 days and either daunorubicin (<60yrs: 90 mg/m2; ≥60yrs: 60 mg/m2) or idarubicin 12 mg/m2 for 3 days. Crenolanib 100 mg TID was administered starting on day 9 until 72 hrs prior to next chemotherapy. Re-induction was permitted for pts with inadequate leukemia cytoreduction. Up to 4 courses of consolidation consisting of 6 doses of high dose cytarabine (HiDAC) (<60yrs: 3 g/m2; ≥60yrs: 1g/m2) was given, with crenolanib starting on day 7. Eligible pts could proceed to allogeneic stem cell transplant (SCT). Crenolanib maintenance therapy was offered for 1 year after HiDAC or -SCT. Safety and tolerability were assessed as well as anti-leukemic activity.
Results:
Demographics: Twenty-six pts (14 females, 12 males) with a median age of 55yrs (range 22-74yrs) have received 7+3 followed by crenolanib; nine (35%) pts were ≥ 60yrs. Five pts had initial WBC >100,000/μL (two pts had WBC >200,000). Three pts had AML following antecedent MDS or MPN.
Mutational Analysis: Twenty-two pts had either FLT3-ITD (n=19) or FLT3-D835 (n=3) mutations. Genomic sequencing revealed multiple concurrent FLT3 activating mutations in four pts. One pt had a dual-activating kinase domain mutation in trans (D835Y+N841T). Three other pts had FLT3-ITD together with N841 (n=2), I836del (n=2), and V592/593 (n=2). Fifteen (60%) pts had FLT3 and NPM1 mutations, and 11 (52%) had FLT3 and DNMT3A mutations. Five pts (24%) had AML with three mutations (FLT3 + NPM1+ DNMT3A). WIT (WT1, IDH1/2, TET2) mutations were present in 11 (52%) pts. Three pts had trisomy 8; one pt each having monosomy 7, trisomy 4, and t(3;18)del(6q)der(3).
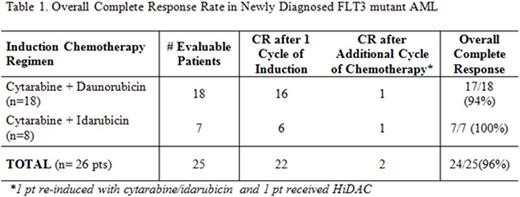
Treatment Outcome: Eighteen (69%) pts received induction with cytarabine+daunorubicin (10 pts at 90 mg/m2, 8 pts at 60 mg/m2) and eight pts (31%) received cytarabine+idarubicin. To date, crenolanib has been well-tolerated in combination with chemotherapy. Six pts required crenolanib dose reductions for periorbital edema (n=2), delayed count recovery (n=1), LFT elevation (n=1), nausea (n=1) and rash (n=1). Out of the twenty-five pts evaluable for response, twenty-two (88%) achieved complete remission (CR) with full count recovery after one cycle of induction. Overall CR/CRi rate was 96% (Table 1).
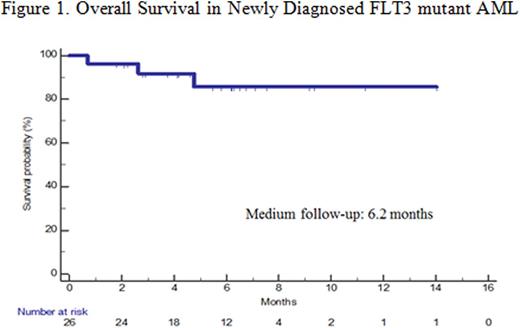
Sixteen pts (10 pts< 60yrs; 6 pts≥ 60yrs) have received a total of 26 cycles of consolidation with HiDAC and crenolanib. Twelve pts (46%) have been bridged to allogeneic SCT. With a median follow up of 6 months, only three pts (all > 60yrs) have relapsed. Overall survival is shown in Figure 1.
Conclusion: Crenolanib, a type I pan-FLT3 TKI, can be safely combined at full doses with cytarabine/daunorubicin or cytarabine/idarubicin induction and HiDAC consolidation chemotherapy for upfront AML therapy. A high CR rate of 88% with full count recovery was observed after one cycle of induction with an overall CR/CRi rate of 96%. HiDAC consolidation and allo SCT could be administered on schedule. Encouraging anti-leukemic activity has been observed with no relapses in FLT3 mutant AML pts <60yrs. Accrual to this trial continues.
Stone:Roche: Consultancy; Sunesis Pharmaceuticals: Consultancy; Xenetic Biosciences: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy; Agios: Consultancy; Amgen: Consultancy; Celator: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy; Novartis: Consultancy; Jansen: Consultancy; Pfizer: Consultancy; ONO: Consultancy; Juno Therapeutics: Consultancy; Merck: Consultancy. Eckardt:Arog: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal