Abstract
Two common genetic modifiers, α-thalassemia and the BCL11A rs1427407 T allele, are observed in approximately one-third of patients with sickle cell anemia (SCA) and are associated with reduced hemolysis and higher hemoglobin F (HbF) levels, respectively.
We investigated the laboratory and clinical effects of α-thalassemia and the BCL11A rs1427407 T allele in the University of Ibadan cohort of SCA patients and replicated our findings in two independent SCA cohorts, University of Illinois at Chicago (UIC) and Walk-Treatment of Pulmonary Hypertension and Sickle cell disease with Sildenafil Therapy (Walk-PHaSST). Alpha-thalassemia status was determined by PCR in all 3 cohorts while the BCL11A rs1427407 genotype was determined by PCR in the Ibadan and UIC cohorts and imputed in the Walk-PHaSST cohort. Comparisons according to genotype were performed using the linear trend test for continuous variables and Cochran's test of linear trend for categorical variables.
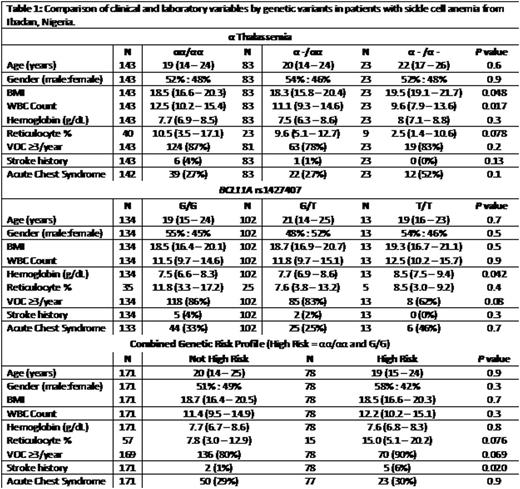
Alpha-thalassemia was observed in 43% of 257 SCA patients from Ibadan and was associated with higher body mass index and lower white blood cell count (Table 1). The BCL11A rs1427407 T allele was observed in 46% of SCA patients from Ibadan and was associated with higher hemoglobin concentration. HbF levels by HPLC were available in 25 patients (12 with the BCL11A rs1427407 T allele) enrolled in a study of low-dose hydroxyurea; these levels were higher in patients with at least one rs1427407 T allele at baseline and progressively during therapy with hydroxyurea 10 mg/kg/day (repeated measures P=0.01).
We defined a high risk genetic group as the absence of α-thalassemia in combination with absence of the BCL11A rs1427407 T allele. This high risk group was observed in 31% of SCA patients from the Ibadan cohort and was associated with a higher reticulocyte percentage (15.0% vs. 7.8%, P=0.08) and a higher prevalence for a history of stroke (6% vs. 1%, P=0.02). The association with stroke history persisted on logistic regression analysis after adjusting for age, gender, and hydroxyurea therapy (OR 9.4, 95%CI: 1.2-72.8; P=0.03). We then replicated the association of this high risk group with markers of hemolysis and with history of stroke in the UIC and Walk-PHaSST SCA cohorts. In the UIC cohort, the high risk group was observed in 34% (92/271) and was also associated with higher reticulocyte counts (13.5% vs. 11.9%, P=0.10) and higher prevalence for stroke history (33% vs. 22%; age, gender, HU-adjusted OR 1.7, 95%CI: 1.0-3.0; P=0.066). In the Walk-PHaSST cohort, this high risk profile was observed in 38% (149/394) and was associated with a higher reticulocyte percentage (9.7% vs. 8.4%, P=0.0005), lower hemoglobin concentration (8.4 vs. 8.8 g/dL; P=0.017), and higher prevalence for stroke history (15% vs. 6%; age, gender, HU-adjusted OR 2.6, 95%CI: 1.3-5.3; P=0.007).
In conclusion, a high risk group of SCA patients, defined by the lack of the protective α-thalassemia and the BCL11A rs1427407 variants, is associated with a higher degree of hemolysis and a higher prevalence of stroke history on cross sectional analysis in three independent cohorts. This high-risk profile may help identify patients to prioritize for hydroxyurea therapy and for closer monitoring strategies for stroke.
Hsu:Sancilio: Research Funding; Astra Zeneca: Consultancy, Research Funding; Purdue Pharma: Research Funding; Gerson Lehman Group: Consultancy; Eli Lilly: Research Funding; Centers for Medicare and Medicaid Innovation: Research Funding; Hilton Publishing: Consultancy, Research Funding; Mast Therapeutics: Research Funding; EMMI Solutions: Consultancy; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal