Abstract
Background: Pracinostat, a potent oral Class I, II, IV histone deacetylase (HDAC) inhibitor, exhibited modest single-agent activity in relapsed AML. Inhibition of histone deacetylation and DNA hypermethylation induces re-expression of silenced genes in myeloid malignancies in a synergistic fashion. A pilot study of pracinostat with azacitidine (AZA) in myelodysplastic syndromes showed the combination to be active and well tolerated (Blood2012;120:3821). We conducted a Phase 2 study to evaluate pracinostat + AZA in patients ≥65 years with AML not eligible for induction chemotherapy. Minimum 1-year survival data were reported previously (Blood 2015;126:453). Here we report long-term survival and response analyses.
Methods: Main eligibility: Age ≥65, untreated de novo or secondary AML, ≥20% bone marrow (BM) blasts, not candidate for intensive therapy due to co-morbidities and/or AML features, intermediate or high-risk cytogenetics, and no prior treatment with HDAC inhibitors or hypomethylating agents. Treatment consisted of pracinostat 60 mg orally 3 days/week on alternate days for 3 weeks and AZA 75 mg/m2 subcutaneously or intravenously daily for 7 days, with cycles repeated every 28 days until disease progression, lack of response or poor tolerability. The primary endpoint was a composite complete response rate (cCR) of CR + CRi+ MLFS by IWG criteria. Response was assessed at the end of Cycles 2, 4, 6 and then every 3 cycles or when clinically indicated. Secondary endpoints included overall response rate (cCR + PR), duration of response and overall survival (OS). Study registered under NCT01912274.
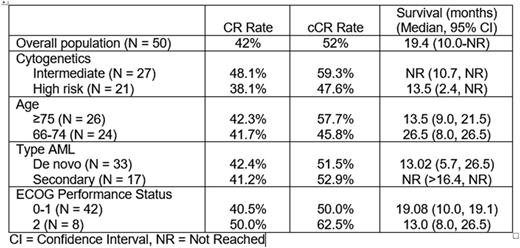
Results: Between December 2013 and December 2014, 50 patients were enrolled at 15 U.S. sites. Baseline characteristics: Median age 75 years (range 66-84 years); 33 de novo and 17 secondary AML; 27 intermediate-risk and 21 high-risk cytogenetics and 2 not known; median BM blasts 40% (range 20%-89%), ECOG performance status 0-1 in 84% and 2 in 16%. The median number of treatment cycles was 6.5 (range 1-24+). A CR was achieved in 21 patients (42%), CRi in 2 (4%), and MLFS in 3 (6%) for a cCR rate of 52%. Median time to BM blasts <5% was 57 days (range 25-243 days) and exceeded 168 days (6 cycles) in 3 patients (12%). Median duration of cCR is 13.2 months (95% CI 10.9-21.5 months), with 6 of 26 patients (23%) continuing on therapy for a periods ranging from 20.5 to 29.5 months. With a median follow-up of 21 months, the median OS is 19.1 months (95% CI, 10.0-not reached). One-year and 2-year OS are 62% and 45%. Non-protocol therapies (NPT) were administered in 23 patients (46%). There was no effect on OS after censoring survival duration at the start of NPT. Response and OS by population subsets are summarized in Table 1. Non hematologic adverse events Grade ≥3 reported in >5% of patients: Fatigue 34%; anorexia 10%, cellulitis, pneumonia, asthenia and sepsis in 8%; urinary tract infection, nausea, back pain, hypoxia, hyponatremia, and syncope in 6%. Grade ≥ 3 hematologic toxicities in> 5% of patients: thrombocytopenia 46%; febrile neutropenia 44%; neutropenia 36%; anemia 30%; pancytopenia 6%. The 30-day and 60-day mortality rates were 2% and 10%.
Conclusions: Pracinostat + AZA led to a high rate of responses in elderly patients with AML. Responses were durable and observed irrespective of age, cytogenetics risk, ECOG performance status, and de novo or secondary AML. Marrow remission was typically achieved early, but prolonged exposure was required in some patients to maximize response. The results compare favorably with historical single-agent AZA data in a similar AML population. A phase 3 study of AZA +/- pracinostat in untreated patients with AML unfit for standard induction chemotherapy is starting.
Response and Survival According to Baseline Characteristics
Response and Survival According to Baseline Characteristics
Atallah:Incyte: Consultancy; CTI biopharma: Consultancy; Novartis: Consultancy; BMS: Consultancy; Pfizer: Other: Grant review; Takeda: Research Funding; Ariad: Honoraria. Arellano:Cephalon: Research Funding. Odenike:Geron: Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi-Aventis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Suneisis: Honoraria, Membership on an entity's Board of Directors or advisory committees; CTI/Baxter: Honoraria, Membership on an entity's Board of Directors or advisory committees; Spectrum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Algeta: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ghalie:MEI Pharma: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal