To the editor:
Although the cause of short stature in children with thalassemia major is not well understood, it is believed to be multifactorial.1 Many different factors including iron overload, intensive use of iron chelators, and gonadal damage may interact, making it difficult to understand each factor’s relative contribution.2-8 It is noteworthy that short stature related to thalassemia major has been found to persist despite major advances in the treatment.1,9
In this study, we evaluated the growth patterns of 245 children and adolescents with thalassemia major (129 males) who were born between 1970 and 2011 and were under regular observation and treatment at the Ospedale Microcitemico, Cagliari, Italy.
In particular, we investigated whether changes that have profoundly modified the course of this disease during recent decades (particularly in relation to the advent of oral iron chelators) have also had an effect on the prevalence of short stature. We also assessed whether the prevalence of iron complications in the pediatric stage had changed over time.
Three birth cohorts were identified as follows:
Group 1 included 64 patients (28 males) born before 1978, when the first iron chelator desferrioxamine was introduced at our center.10
Group 2 included 122 patients (71 males) born between 1978 and 1995, when a hypertransfusional regimen (pretransfusional hemoglobin 11 g/dL, posttransfusional hemoglobin 14-15 g/dL) was commonly adopted. At that time, desferrioxamine was first administered in high doses (up to 100 mg/kg) and initiated at the same time or soon after the start of transfusion therapy. Over the years, this regimen was administered progressively later and in lower doses.11
Group 3 consisted of 59 patients (29 males) born between 1996 and 2011. At the last evaluation, 34 patients were younger than age 12 years, 11 patients were between ages 12 and 16 years, and 12 patients were aged 16 years or older. This group had mostly been treated with oral chelators since childhood and/or adolescence. In addition, they were the first to benefit from the change to the current transfusional regimen (pretransfusional hemoglobin, 9-10.5 g/dL; posttransfusional hemoglobin, 12.5-13.5 g/dL).12
Although our study included all patients born after 1995 who received regular follow-up visits at our center, the patients belonging to the other birth cohorts were randomly selected, on an alphabetical basis, until a double number of subjects per cohort was reached.
Growth was assessed by monitoring changes in standing height every 6 ± 2 months with a Harpenden stadiometer and expressed as height-standard deviation score (h-SDS).9 For patients born after 1995, height was also related to a target calculated by sex-adjusted midparental height.
In total, 145 (59%) of 245 patients were of short stature (h-SDS < −2), and similar proportions were found between the sexes by age 18 years.
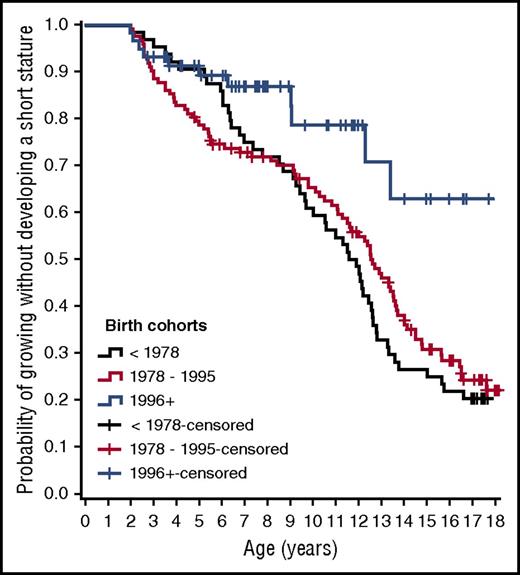
Short stature was observed in 51/64 (80%) children born before 1978, 83/122 (68%) born between 1978 to 1995, and 11/59 (19%) born in 1996 or later. At 16 years of age, approximately 35% of those born after 1995 had a stature less than −2 h-SDS, and short stature was observed in 78% of children born before 1978 and in 75% of those born between 1978 and 1995 (Figure 1). The heights of all of the subjects born between 1996 and 2011 fell between the third and 97th percentiles for anticipated adult height.
Kaplan-Meier analysis showing the probability of growing without developing short stature (h-SDS < −2) in children with thalassemia major in the 3 cohorts of patients studied.
Kaplan-Meier analysis showing the probability of growing without developing short stature (h-SDS < −2) in children with thalassemia major in the 3 cohorts of patients studied.
We analyzed the ages at which the patients reached a short stature, using a Cox proportional hazards model. The time to the event outcome was defined as the age when patients eventually fell below an h-SDS of −2, whereas the factors considered were as follows: sex, age at start of transfusions, age at start of chelation therapy, intensive and/or early chelation with desferrioxamine, use of oral chelators, chronic hepatitis C, and iron-related complications. In addition, we analyzed the ages at which endocrinopathies eventually occurred, also using Cox proportional hazards models. Variations of the effects of time were considered (as a binary covariate at 5 years of age, based on the observation of the survival curves). The results are listed in Table 1.
Factors influencing age at short stature
| Conditions . | Age at short stature* . | |||
|---|---|---|---|---|
| Before 5 y old . | After 5 y old . | |||
| H.R. . | P value . | H.R. . | P value . | |
| Birth cohort | ||||
| <1978 | 0.387 | .037 | n.s. | n.s. |
| 1978-1995 | ref. | ref. | ||
| >1995 | n.s. | n.s. | 0.396 | .032 |
| Age at start of chelation therapy, y | 0.796 | .031 | n.s. | n.s. |
| Conditions . | Age at short stature* . | |||
|---|---|---|---|---|
| Before 5 y old . | After 5 y old . | |||
| H.R. . | P value . | H.R. . | P value . | |
| Birth cohort | ||||
| <1978 | 0.387 | .037 | n.s. | n.s. |
| 1978-1995 | ref. | ref. | ||
| >1995 | n.s. | n.s. | 0.396 | .032 |
| Age at start of chelation therapy, y | 0.796 | .031 | n.s. | n.s. |
A stepwise Cox proportional hazards model was fitted for age at h-SDS less than −2. The model included sex as a stratifying variable and the following variables at first step: birth cohorts, age at start of transfusions, age at start of chelation therapy, intensive and/or early chelation with desferrioxamine, use of oral chelators, chronic hepatitis C, primary amenorrhea, hypogonadism, hypoparathyroidism, hypothyroidism, and diabetes.
H.R., hazard ratio; n.s., not significant; ref., reference category.
Short stature was defined as h-SDS less than −2.
As shown in Figure 1 and Table 1, with respect to the 1978 to 1995 birth cohort, the patients born before 1978 and after 1995 were less prone to develop short stature before age 5 years (although not significantly for the after-1995 cohort: P = .086; hazard ratio, 0.458). For patients born before 1978, this effect was no longer present after age 5 years. This phenomenon may be a result of early hypothalamic/pituitary damage induced by iron overload, and/or by the toxic effects of iron deposition in tissues.13-16 The high number of patients in the 1978 to 1995 birth cohort with short stature in the first years of life confirmed the detrimental role of the early start of iron chelation (commonly employed at that time), as previously suggested.7,17-19
In both of the cohorts born before 1978 and between 1978 and 1995, the early start of chelation treatment was not the only risk factor; also important were the high doses of desferrioxamine administered in the first years of life, confirming previous findings (supplemental Table 1, available on the Blood Web site).7,17-19
Among the children born in or after 1996, 41 received deferasirox before age 10 years for at least 3 years (mean ± standard deviation of start age was 4.3 ± 1.7 years, corresponding to mean h-SDS of −0.79 ± 0.77). Their last h-SDS on deferasirox at 9.0 ± 4.2 years was −0.9 ± 0.8 (paired t-test P = .08), with 15 children presenting an increase in h-SDS and 27 presenting a decrease.
As found by Aydinok et al in 2012, although the risk of developing short stature was lower among children receiving the oral chelator, absolute changes in h-SDS in patients receiving deferasirox vs those receiving desferrioxamine were not significantly different.19 It is also noteworthy that, although remaining within the normal range, h-SDS tended to decrease over the years, even in most children receiving deferasirox. We observed a decrease of stature in 6 of the 7 adolescents who received oral iron chelators after age 10 years for 3 years or more (from mean h-SDS of −0.9 ± 0.8 to −1.31 ± 0.59; paired t-test P = .05; 6 males and 1 female, deferiprone in 2 cases). Therefore, an eventual positive effect of oral chelators on growth does not seem to be sufficient when started after age 10 years.
Although primary amenorrhea in females and hypogonadism in males were not found to be significant risk factors for short stature, we observed a lower prevalence of primary amenorrhea and male hypogonadism in younger cohorts, in accordance with other reports.4,20-22
Spontaneous menarche occurred in 42% (15/36) of patients born before 1978, in 69% (35/51) born between 1978 and 1995, and in 90% (9/10) in the last cohort. Moreover, for the first time, we observed a significant decrease in the age at menarche in later cohorts with respect to earlier ones (P = .001, Kaplan-Meier analysis with log-rank test).22
The prevalence of each endocrinopathy according to birth cohort is shown in supplemental Table 2.
The presence of oligomenorrhea even in the youngest patients may indicate that the iron-related impairment of hypothalamic/pituitary functions occurred early and at a low iron load, as previously speculated.10-13 Adequate chelation earlier in life might well prevent more severe degrees of glandular malfunction. In this regard, the risk for primary amenorrhea was lower among girls who started taking oral chelators before puberty (P = .006; supplemental Table 3). None of the patients born after 1995 developed hypothyroidism, hypoparathyroidism, or diabetes before age 18 years.
These results showed that adherence to modern transfusion and iron chelation protocols and avoidance of iron chelator overdosage clearly reduced the risk for short stature and may have potentially enhanced endocrine development in children with homozygous β thalassemia. Longer-term studies are required to confirm whether these favorable results will continue to be observed in late adolescents and young adults.
The online version of this article contains a data supplement.
Authorship
Contribution: R.O., F.D., and S.L. designed the research study, analyzed the data, and wrote the paper. V.O., A.Z., and A.D. gave a substantial contribute to the acquisition of data. M.L.F., G.B.L., M.M., P.M., and C.D. contributed to acquiring, analyzing, and interpreting the data. All authors revised the paper critically and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raffaella Origa, University of Cagliari, Ospedale Pediatrico Microcitemico, Via Jenner s.n. 09121 Cagliari, Italy; e-mail: raffaella.origa@unica.it.
References
Author notes
R.O. and F.D. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal