In this issue of Blood, Urrutia et al identified pericyte as a novel erythropoietin (EPO)-producing cell type in the brain. The brain pericytes function as oxygen sensors and respond to hypoxia. They are regulated by hypoxia-inducible factor (HIF)-2 and prolyl-4-hydroxylase (PHD) 2 and 3.1
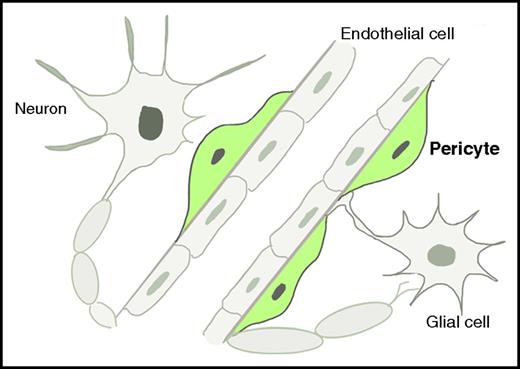
Schematic illustration of brain EPO-producing cells. These cells comprise neurons, glial cells (including astrocytes and oligodendrocytes), endothelial cells, and pericytes. The pericytes, which are highlighted in green, are revealed by Urrutia et al to be the main EPCs in the brain under hypoxic conditions.
Schematic illustration of brain EPO-producing cells. These cells comprise neurons, glial cells (including astrocytes and oligodendrocytes), endothelial cells, and pericytes. The pericytes, which are highlighted in green, are revealed by Urrutia et al to be the main EPCs in the brain under hypoxic conditions.
EPO is the principle cytokine for the production of red blood cells, especially under hypoxic conditions. During mammalian fetal developmental, EPO is mainly generated in the liver, which is also one of the major organs for fetal erythropoiesis. After birth, the majority of EPO is produced in the kidneys. Main renal EPO-producing cells (EPCs) are peritubular interstitial cells that express markers including platelet-derived growth factor receptor-β (PDGFRB) and neuro-glial antigen 2 (NG2). These markers are characteristically expressed in pericytes and neuronal cells.2 The developmental association between kidney EPCs and neuronal cells has led to the discovery of EPCs in the brain >2 decades ago.3 To date, brain EPCs are found to include neurons, glial cells, and endothelial cells. Using elegant mouse genetics and RNA fluorescence in situ hybridization (FISH) approaches, Urrutia et al now identify pericyte as another, and the major, type of EPC in the brain, especially under the hypoxic conditions (see figure).
In the kidneys, the hypoxic induction of EPO is controlled by HIF-2, which is composed of an oxygen-sensitive α-subunit and a constitutively expressed β-subunit. HIF-α is regulated by oxygen, iron, and PHD domain-containing proteins 1, 2, and 3. PHD proteins hydroxylate HIF-α, which leads to its binding of von Hippel-Lindau (VHL) E3 ubiquitin ligase and subsequent degradation by the proteasome. As a proof-of-concept, Urrutia et al first investigated the PHD/VHL-HIF-EPO pathway in a mouse model with VHL knockout specifically in NG2-expressing cells (NG2-Vhl−/−). These mice developed polycythemia with increased plasma EPO concentration. Although NG2 is expressed in various cell types in addition to pericytes, statistically significant Epo transcripts were only observed in the brain, kidneys, and bone. Among these tissues, brain showed the most pronounced fold increase. In addition, the authors performed high-resolution RNA FISH and convincingly demonstrated that the pericyte is the main source of EPO in the brain in NG2-Vhl−/− mice by showing that a majority (60%) of Epo transcript-positive cells are also positive for Pdgfrb transcript in these mice.
These intriguing data reveal the pericyte as a major type of EPC in the brain. However, the relative contribution of EPO produced by the brain pericytes to circulating EPO level remains unclear. Although this may not be easy to investigate in normoxic conditions given the impermeable nature of the blood–brain barrier to EPO, Urrutia et al show that, under hypoxic situations, pericytes that coexpress EPO and PDGFRB represent the major EPCs in the brain. They demonstrate that when the wild-type brain pericytes are exposed to 8% oxygen, the Epo and Pdgfrb double-positive cells represent 25% to 45% of the total number of Epo-positive cells. When they induce the wild-type mice to anemic hypoxia through phlebotomy, this number rises to 70%. The rest of the Epo-positive cells are presumably other types of EPCs in the brain as mentioned above.
EPO production in the kidneys is negatively regulated by PHD2, whereas combined inactivation of all 3 PHD proteins is needed to induce EPO in hepatocytes. In the brain pericytes, PHD2 and PHD3 are now shown to be required to reduce HIF-2. Using a similar genetic approach, the authors show that NG2-Phd2−/−Phd3−/− mice exhibit polycythemia that can be reverted in NG2-Phd2−/−Phd3−/−Hif2a−/− mice. The involvement of various PHD proteins in different tissues indicates cell type–specific regulation of HIF activity and EPO production. It is also possible that the PHD proteins play a distinct function in addition to EPO production. In the brain, this could be that the combined activities of PHD proteins facilitate the delivery of the pericyte-produced EPO to the systemic circulation through their influences on blood–brain barrier permeability. Consistent with this hypothesis, NG2-Phd1−/−Phd2−/−Phd3−/− triple knockout mice were found to have increased plasma EPO than NG2-Phd2−/−Phd3−/− mice. However, the brain Epo mRNA or EPO protein levels showed no differences between these 2 groups of mice.
EPO production by the brain pericytes has perhaps more significant implications than the contribution to circulating EPO during hypoxia. The EPO receptor (EPO-R) is expressed in neural progenitor cells, neurons, glial cells, and endothelial cells. EPO has a well-documented role in neuroprotection, especially in ischemic brain injury.4 Targeted knockout of EPO-R in the mouse brain reduces neural cell proliferation and impairs poststroke neurogenesis.5 EPO injection also protects brain injury in vivo.6 The most compelling evidence of EPO in neuroprotection comes from the fact that EPO derivatives lacking erythropoiesis stimulating activity still conserve the neuroprotective effects in animal models of brain injury.7 In addition to EPO production, endothelial cells, one of the critical components of neutral stem cell niche, are responsive to EPO stimulation to contribute to neurogenesis and neuroprotection.4 EPO promotes endothelial cell migration, proliferation, production of nitric oxide, and angiogenesis.8 In this respect, the direct interaction of pericytes with endothelial cells could provide immediate EPO stimulation to endothelial cells for their neuroprotective function. This could also circumvent the difficulty of circulating EPO to reach the injury sites in the brain given the robust EPO generation by the brain pericytes under hypoxic conditions.
Although the significance of the brain pericytes in EPO production under normoxic and hypoxic conditions in human remains to be investigated, this study advances the field and establishes the basis for future studies on the collaborative roles of various brain EPCs in oxygen sensing, EPO production, and neuroprotection.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal