To the editor:
We identified very primitive CD34-negative (CD34−) severe combined immunodeficiency–repopulating cells (SRCs) in human cord blood (CB) using the intrabone marrow injection method1 and provided a new concept for the hierarchy in the human hematopoietic stem cell (HSC) compartment.2,3 However, the incidence of CD34− SRC in 13 lineage-negative (Lin−) CD34− cells (1/25 000) was very low.1 We then developed a high-resolution purification method for CD34− SRCs using 18Lin-specific antibodies, which can enrich CD34− SRC at the 1/1000 level in 18Lin−CD34− fraction.4 In addition, we recently identified CD133 as a positive marker for CD34− as well as CD34+ SRCs.5 A limiting dilution analysis (LDA) demonstrated that CD34+/− SRCs were enriched to ∼1/100 and 1/140 in 18Lin−CD34+/−CD133+ fractions, respectively.3,5 Our goal is to purify CD34− HSCs to the single-cell level, in order to further elucidate the details of the characteristics of human CB-derived CD34− HSCs. It was therefore necessary to identify additional specific positive/enrichment markers for CD34− HSCs. Until recently, several investigators have reported the usefulness of CD49f, CD93, and CD82 in the enrichment of human CB- and bone marrow (BM)-derived CD34+/− HSCs.6-8
Using the above-mentioned 18Lin−CD34− cell population, we extensively analyzed the expression of candidate markers by flow cytometry (FCM). Finally, we demonstrated that a glycosylphosphatidylinositol-anchored protein GPI-80, which had originally been reported to regulate neutrophil adherence and migration,9,10 was also expressed on human full-term CB-derived 18Lin−CD34+CD38− and 18Lin−CD34− cells. Interestingly, human CB-derived CD34− SRCs were highly enriched in the 18Lin−CD34−GPI-80+ cell fraction.
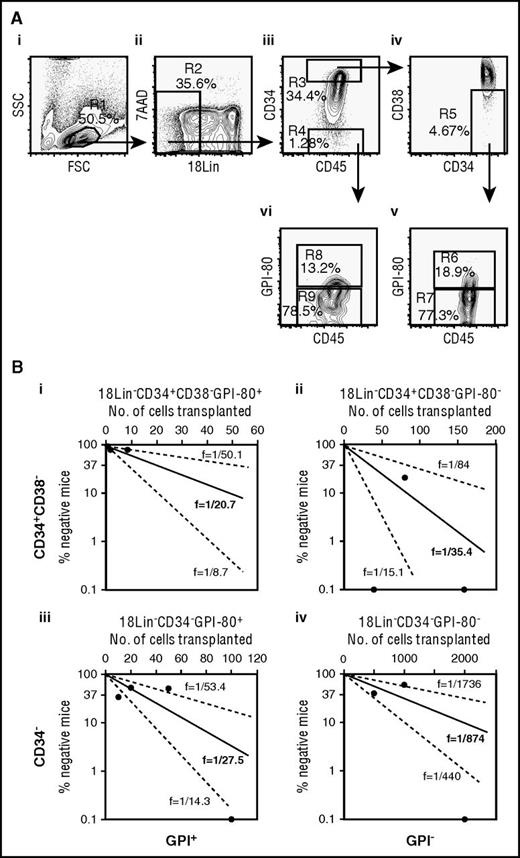
Details of the experimental methods can be found in the supplemental Data, available on the Blood Web site. First, we extensively explored candidate positive/enrichment markers, which could be useful for the efficient purification of rare CB-derived CD34− SRCs (HSCs), including known HSC markers and various adhesion molecules, using highly purified CB-derived 18Lin−CD34+/− cells4 by multicolor FCM. Out of the 25 molecules that were analyzed, GPI-80, c-kit, CD90, CXCR4, CD49f, and CD93 were expressed on both types of cells. The 18Lin−CD34+/− cells could be subdivided into positive and negative fractions (supplemental Figure 1A). In order to select an appropriate marker from these candidate molecules, we back-gated the positive fractions of these markers in 18Lin−CD34− cells into FSC/SSC scattergrams. As clearly seen in supplemental Figure 1B, the distribution of c-kit+, CD90+, CXCR4+, CD49f+, and CD93+ cells in the 18Lin−CD34− cells was scattered inside and around the blast window. In contrast, most of the 18Lin−CD34−GPI-80+ cells were concentrated in the low FSC blast window. Based on these data, we selected GPI-80 as a candidate positive/enrichment marker for human CB-derived CD34− SRCs (HSCs). We then purified 18Lin−CD34+CD38−GPI-80+ and 18Lin−CD34−GPI-80+ cells, as shown in Figure 1Av-vi. Approximately 5% to 30% of the 18Lin−CD34+CD38− and 18Lin−CD34− cells expressed GPI-80. These 18Lin−CD34+CD38−GPI-80+/− and 18Lin−CD34−GPI-80+/−cells were then sorted for the following in vivo as well as in vitro experiments.
(A) The representative FACS profiles of CB-derived 18Lin−CD34+CD38−GPI-80+/− and 18Lin−CD34−GPI-80+/− cells (i to vi) and (B) a comparison of the frequency of SRCs in CB-derived 18Lin−CD34+CD38−GPI-80+/− and 18Lin−CD34−GPI-80+/− cells (i to iv). (A) (i) The forward scatter/side scatter (FSC/SSC) profile of immunomagnetically separated Lin− cells. The R1 gate was set on the blast-lymphocyte window. (ii) The R2 gate was set on the 18Lin− living cells. (iii) The 18Lin− living cells (R2) were subdivided into 2 fractions: 18Lin−CD45+CD34+ (R3) and 18Lin−CD45+CD34− (R4) cells, according to their CD34 expression levels. CD34+/− cells were defined as follows: the CD34+ fraction contains cells expressing maximum antigen-presenting cell fluorescence intensity (FI) to the 5% level of FI. The CD34− level of FI was determined based on Fluorescence Minus One controls. (iv) The 18Lin−CD45+CD34+ cells residing in the R3 gate were further subdivided into CD38− (R5) cells, according to their CD38 expression levels. The CD38− level was defined as <15% of the maximum PE-Cy7 FI. (v) The 18Lin−CD45+CD34+CD38− cells residing in the R5 gate were further subdivided into 2 fractions: 18Lin−CD45+CD34+CD38−GPI-80+ (R6) and GPI-80− (R7) cells, according to their GPI-80 expression levels. GPI-80+/− cells were defined as follows: the GPI-80− level of FI was determined based on Fluorescence Minus One controls, and remaining cells were defined as GPI-80+ cells. (vi) The R4-gated cells were further subdivided into 2 fractions: 18Lin−CD45+CD34−GPI-80+ (R8) and GPI-80− (R9) cells. The definitions of GPI-80+/− cells are the same as abovementioned. As shown in (v) and (vi), the percentages of GPI-80+ cells in the CD34+ (R6) and CD34− (R8) fractions ranged from 5.4% to 19.9% (mean 11.8%, n = 6) and 5.4% to 29.9% (mean 14.2%, n = 6), respectively. (B) Various numbers of 18Lin−CD34+CD38−GPI-80+ cells (1, 2, and 8, n = 32) (i), 18Lin−CD34+CD38−GPI-80− cells (40, 80, and 160, n = 11) (ii), 18Lin−CD34−GPI-80+ cells (10, 20, 50, 100, 200, and 400, n = 26) (iii), and 18Lin−CD34−GPI-80− cells (500, 1000, and 2000, n = 15) (iv) were transplanted into NOG mice. The human CD45+ cell repopulation in the mouse BM was analyzed by FCM at 8 weeks after transplantation. The frequency of SRC was 1 per 20.7 in 18Lin−CD34+CD38−GPI-80+ cells, 1 per 35.4 in 18Lin−CD34+CD38−GPI-80− cells, 1 per 27.5 in 18Lin−CD34−GPI-80+ cells, and 1 per 874 in 18Lin−CD34−GPI-80− cells. The middle solid line represents the estimated weighted mean frequency (fWM). The lower and upper dotted lines represent the 95% confidence interval of fWM. The detailed LDA data are presented in supplemental Table 2.
(A) The representative FACS profiles of CB-derived 18Lin−CD34+CD38−GPI-80+/− and 18Lin−CD34−GPI-80+/− cells (i to vi) and (B) a comparison of the frequency of SRCs in CB-derived 18Lin−CD34+CD38−GPI-80+/− and 18Lin−CD34−GPI-80+/− cells (i to iv). (A) (i) The forward scatter/side scatter (FSC/SSC) profile of immunomagnetically separated Lin− cells. The R1 gate was set on the blast-lymphocyte window. (ii) The R2 gate was set on the 18Lin− living cells. (iii) The 18Lin− living cells (R2) were subdivided into 2 fractions: 18Lin−CD45+CD34+ (R3) and 18Lin−CD45+CD34− (R4) cells, according to their CD34 expression levels. CD34+/− cells were defined as follows: the CD34+ fraction contains cells expressing maximum antigen-presenting cell fluorescence intensity (FI) to the 5% level of FI. The CD34− level of FI was determined based on Fluorescence Minus One controls. (iv) The 18Lin−CD45+CD34+ cells residing in the R3 gate were further subdivided into CD38− (R5) cells, according to their CD38 expression levels. The CD38− level was defined as <15% of the maximum PE-Cy7 FI. (v) The 18Lin−CD45+CD34+CD38− cells residing in the R5 gate were further subdivided into 2 fractions: 18Lin−CD45+CD34+CD38−GPI-80+ (R6) and GPI-80− (R7) cells, according to their GPI-80 expression levels. GPI-80+/− cells were defined as follows: the GPI-80− level of FI was determined based on Fluorescence Minus One controls, and remaining cells were defined as GPI-80+ cells. (vi) The R4-gated cells were further subdivided into 2 fractions: 18Lin−CD45+CD34−GPI-80+ (R8) and GPI-80− (R9) cells. The definitions of GPI-80+/− cells are the same as abovementioned. As shown in (v) and (vi), the percentages of GPI-80+ cells in the CD34+ (R6) and CD34− (R8) fractions ranged from 5.4% to 19.9% (mean 11.8%, n = 6) and 5.4% to 29.9% (mean 14.2%, n = 6), respectively. (B) Various numbers of 18Lin−CD34+CD38−GPI-80+ cells (1, 2, and 8, n = 32) (i), 18Lin−CD34+CD38−GPI-80− cells (40, 80, and 160, n = 11) (ii), 18Lin−CD34−GPI-80+ cells (10, 20, 50, 100, 200, and 400, n = 26) (iii), and 18Lin−CD34−GPI-80− cells (500, 1000, and 2000, n = 15) (iv) were transplanted into NOG mice. The human CD45+ cell repopulation in the mouse BM was analyzed by FCM at 8 weeks after transplantation. The frequency of SRC was 1 per 20.7 in 18Lin−CD34+CD38−GPI-80+ cells, 1 per 35.4 in 18Lin−CD34+CD38−GPI-80− cells, 1 per 27.5 in 18Lin−CD34−GPI-80+ cells, and 1 per 874 in 18Lin−CD34−GPI-80− cells. The middle solid line represents the estimated weighted mean frequency (fWM). The lower and upper dotted lines represent the 95% confidence interval of fWM. The detailed LDA data are presented in supplemental Table 2.
Using the 4 above-mentioned cell fractions, we performed an LDA to demonstrate the frequency of the CD34+/− SRCs. As shown in Figure 1Bi-iv and supplemental Table 2, the incidences of SRCs in these 4 cell fractions were as follows: 1/20.7 in 18Lin−CD34+CD38−GPI-80+ cells; 1/35.4 in 18Lin−CD34+CD38−GPI-80− cells; 1/27.5 in 18Lin−CD34−GPI-80+ cells; and 1/874 in 18Lin−CD34−GPI-80− cells. These results clearly demonstrated that GPI-80 expression highly purified the CD34− SRCs and achieved a purification level of ∼1000 times higher than the initial incidence (1/25 000) of CD34− HSCs.1 This purification level (1/27.5) is the highest to date.
We next analyzed the long-term multilineage reconstitution abilities of CD34+CD38−GPI-80+ and CD34−GPI-80+ SRCs. In these experiments, all of the mice that received transplants of 200 18Lin−CD34+CD38−GPI-80+ cells containing 9.7 SRCs and 200 18Lin−CD34−GPI-80+ cells containing 7.3 SRCs showed distinct human cell repopulation at 20 weeks after transplantation (supplemental Table 3). The level of human cell repopulation ranged from 9.3% to 84.7% (median, 66.2%) for 18Lin−CD34+CD38−GPI-80+ cells and from 0.7% to 52.8% (median, 31.9%) for 18Lin−CD34−GPI-80+ cells.
To further evaluate the functional differences between the CD34+CD38−GPI-80+ and CD34−GPI-80+ SRCs, we studied their multilineage reconstitution abilities in the BM, peripheral blood, spleen, and thymus, in the above-mentioned NOG mice. The results of the repopulation patterns are precisely shown in supplemental Figure 2. An analysis of the BM of the 2 representative mice that received either (supplemental Figure 2A) CD34+CD38−GPI-80+ SRC or (supplemental Figure 2B) CD34−GPI-80+ SRC demonstrated that both of the SRCs have an in vivo differentiation capacity that was comparable to that of CD34+ stem/progenitor cells, CD19+ B-lymphoids, CD33+ myeloids, CD11b+ monocytes, CD235a+ erythroid, and CD41+ megakaryocytic lineages. The CD56+ NK cells were detected in the spleen in both NOG mice. The CD3+ T cells were also detected in the thymi in both NOG mice. These results confirmed that both the CD34+CD38−GPI-80+ and the CD34−GPI-80+ SRCs had multilineage differentiation potential.
Next, we cocultured CB-derived 18Lin−CD34+CD38−GPI-80+/− and 18Lin−CD34−GPI-80+/− cells with human BM-derived mesenchymal stromal cells (abbreviated as DP MSCs).11 As shown in supplemental Figure 3, both 18Lin−CD34+CD38−GPI-80+ and 18Lin−CD34−GPI-80+ cells could generate 12Lin−CD45RA−CD34+CD38−GPI-80+/− (abbreviated as 34+38−80+/−) cells. However, 18Lin−CD34+CD38−GPI-80− and 18Lin−CD34−GPI-80− cells could only generate 34+38−80− cells. Moreover, the former 34+38−80+/− cells showed distinct SRC activities. All of the recipient mice (6/6) showed multilineage human cell repopulation (supplemental Table 4). In contrast, the latter 34+38−80− cells showed weak SRC activities. In other words, some of the recipient mice (3/6 for the 18Lin−CD34+CD38−GPI-80− cell-derived 34+38−80− cells and 1/6 for the 18Lin−CD34−GPI-80− cell-derived 34+38−80− cells) were repopulated with human cells. However, the repopulation rates were low in comparison with their GPI-80+ counterparts. All 4 types of input cells generated 12Lin−CD45RA−CD34−GPI-80− (abbreviated as 34−80−) cells (supplemental Figure S3). However, none of the mice that received these 34−80− cells were repopulated with human cells (supplemental Figure 3 and supplemental Table 4). Overall, CD34+/−GPI-80+ SRCs could generate/maintain CD34+GPI-80+/− SRCs, but not CD34−GPI-80+/− SRCs in the cocultures with DP MSCs. In contrast, CD34+/−GPI-80− SRCs could not generate/maintain CD34+/−GPI-80+ SRCs in cocultures with DP MSCs. They could only generate/maintain CD34+GPI-80− SRCs in cocultures with DP MSCs. These results suggest that CD34+/−GPI-80+ SRCs seem to be more immature than CD34+/−GPI-80− SRCs in the human HSC hierarchy. In other words, GPI-80 defines human CB-derived primitive HSCs that can maintain SRC activity in cocultures with DP MSCs.
In conclusion, these observations clearly demonstrated that GPI-80 is a useful enrichment marker for the high-level purification of human CB-derived CD34+/− SRCs (HSCs). GPI-80 has very recently been reported as a positive marker for human fetal liver hematopoietic stem/progenitor cells (HSPCs), and only CD34+CD38lo/−CD90+GPI-80+ cells have shown SRC activity.12 Because human placenta/CB-derived HSPCs reflect fetal hematopoiesis,13-15 it is conceivable that GPI-80 is expressed on CB-derived primitive HSCs. From another point of view, leukemia-initiating cells (LICs) were detected in the CD34− cell fractions of leukemia patients.16-18 In order to eradicate these chemotherapy-resistant CD34− LICs, it is necessary to identify useful markers as targets for molecular-targeting therapy. Thus, it would be interesting to analyze whether these CD34− LICs express GPI-80. It is also interesting to note that both GPI-80 and CD133 were correlated with the polarization and migration of leukocytes and HSPCs.9,10,19,20 Because our goal is to purify CD34+/− HSCs to the single-cell level in order to elucidate key molecules/signals for maintaining their stemness, the next step is to combine GPI-80 with CD133.5 Using these 2 markers, we are now working to develop a method that achieves the ultra-high-resolution purification of human CB-derived CD34+/− SRCs (HSCs).21
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors are grateful to the Japanese Red Cross Kinki Cord Blood Bank for providing the CB samples used in this study. The authors also thank Kyowa Hakko Kirin Company (Tokyo, Japan) for providing the various growth factors used in this study.
This work was supported by Grants-in-Aid for Scientific Research C (grant 24591432) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan; a grant from the Strategic Research Base Development program for Private Universities from the MEXT; MEXT-Supported Program for the Strategic Research Foundation at Private Universities; a grant from the Terumo Life Science Foundation; and a grant from SENSHIN Medical Research Foundation.
Contribution: Y.M. conceived the study and designed the experiments, provided study material, performed all of the experiments, collected and/or assembled data, performed the data analysis, and interpreted the results; K.S., H.K., R.N., and T.F. provided study material and contributed to some of the experiments; and Y.S. was involved in the conception and design, directed the project, provided financial and administrative support, participated in the data analysis and the interpretation of the results, wrote the manuscript, and gave final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshiaki Sonoda, Department of Stem Cell Biology and Regenerative Medicine, Graduate School of Medical Science, Kansai Medical University, Hirakata, Osaka, 573-1010, Japan; e-mail: sonoda@hirakata.kmu.ac.jp.