In this issue of Blood, Rauch et al provide evidence for a novel mechanism to explain a fundamental yet enigmatic observation that has plagued hematologists for decades: the decline in nonleukemic hematopoiesis in the bone marrow of patients with acute myeloid leukemia (AML). The authors found that high expression of the thrombopoietin (TPO) receptor MPL on AML blasts predicts neutropenia and thrombocytopenia, and that AML blasts expressing high levels of MPL deplete TPO in cell culture and in mouse models. Rather than crowding out normal bone marrow hematopoietic stem cells (HSCs), might MPL-expressing AML blasts impair hematopoiesis by stealing the cytokine TPO?1
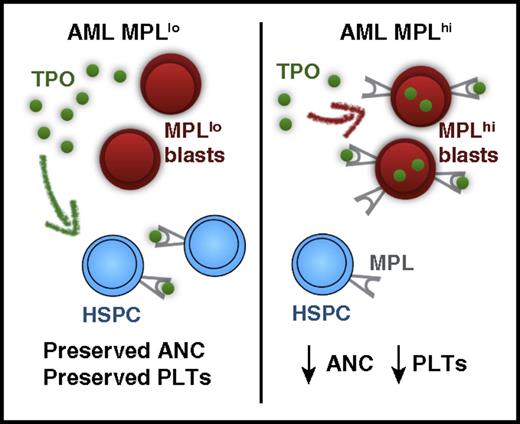
Proposed mechanism for MPL scavenging of TPO leading to neutropenia and thrombocytopenia in AML. PLTs, platelets. The figure has been adapted from Figure 2 in the article by Rauch et al that begins on page 2253.
Proposed mechanism for MPL scavenging of TPO leading to neutropenia and thrombocytopenia in AML. PLTs, platelets. The figure has been adapted from Figure 2 in the article by Rauch et al that begins on page 2253.
Most patients with AML present with cytopenias in 1 or more cell lineages. However, the mechanisms by which AML impairs normal hematopoiesis have remained elusive. Is it simple competition for nutrients or space? Why do some patients with a bone marrow full of blasts have relatively preserved blood counts, whereas others experience life-threatening cytopenias with far less bone marrow involvement?
Previous studies reveal multiple mechanisms by which AML blasts might induce cytopenias, including inhibition of normal HSC proliferation and differentiation.2,3 Leukemic cells also alter the stromal microenvironment, creating abnormal malignant niches that sequester normal hematopoietic progenitor cells and fail to support normal hematopoiesis.4 Recent work demonstrates that in the presence of leukemia, expression of the transcription factor Egr3 in normal HSCs plays a key role in inhibiting normal HSC proliferation and promoting HSC quiescence.3 Bone marrow plasma from leukemic hosts also suppresses proliferation of hematopoietic stem and progenitor cells, and previous hypotheses suggested that leukemia cells secrete as yet unidentified cytokines to suppress normal HSC function.3 As an alternative, might bone marrow plasma from some leukemic hosts be deficient in a cytokine found in normal plasma? The current study provides evidence for this latter model.
Rauch et al found no correlation between peripheral blood counts (hemoglobin and platelets) and bone marrow blast infiltration in 2 independent cohorts of patients with newly diagnosed AML. This simple observation challenges the prevailing assumption that high bone marrow blast counts are the primary driver of cytopenias in AML. In 1 AML patient cohort, there was a weak but statistically significant negative correlation between bone marrow infiltration and absolute neutrophil count (ANC), suggesting that higher blast occupancy might play at least some role in promoting neutropenia. The authors then examined the relationship between platelet counts and serum concentrations of TPO in AML patients.
Current models propose that TPO levels are regulated both at the level of production and clearance.5 At the level of TPO production, binding of senescent, desialylated platelets to the Ashwell-Morell receptor induces hepatic expression of TPO, triggering thrombopoiesis. At the level of clearance, platelet and megakaryocyte expression of the TPO receptor MPL lead to MPL-mediated TPO endocytosis and TPO removal. The balance of these mechanisms enables TPO and platelet homeostasis in normal individuals.
Rauch et al showed no significant correlation between TPO serum concentration and platelet count in AML patients. This lack of correlation was driven by AML patients with severe thrombocytopenia who had lower than expected TPO serum levels. Given the models of TPO homeostasis described previously, severe thrombocytopenia should result in increased circulating TPO. However, consistent with prior studies,6 the authors found that selected AML patients with severe thrombocytopenia and low TPO levels had high expression of the TPO receptor MPL on AML blasts. Might high MPL levels on AML blasts clear TPO and thereby lead to cytopenias?
From a mechanistic perspective, the authors provided evidence that AML blasts with high expression of MPL (MPLhi) could deplete TPO levels in tissue culture. Xenotransplantation of MPLhi AML cells vs MPLlo AML cells also demonstrated in vivo depletion of TPO by MPLhi AML cells in a humanized TPO mouse model.
In microarray analysis of bone marrow from AML patients, expression of MPL and TPO pathway genes were enriched in patients with severe thrombocytopenia (0-20) at diagnosis compared with patients with platelet counts of 50 to 100. Notably, patients with MPLhi AML blasts had significantly lower platelet counts and ANC (see figure). Consistent with previous observations,7 mean MPL expression was also higher in t(8;21) than in other AML cytogenetic and molecular subgroups. Accordingly, platelet counts and ANC were lower in t(8;21) AML. However, no difference in hemoglobin concentration was seen between patients with MPLhi vs MPLlo AML, or between t(8;21) and other AML subgroups, suggesting that anemia in AML patients is driven by mechanisms independent of AML MPL expression.
This provocative work raises multiple questions. Given the in vivo depletion of TPO by MPLhi blasts in a mouse model, do AML patients with MPLhi t(8;21) blasts have lower serum levels of TPO? Why is high MPL expression on AML blasts associated with thrombocytopenia and neutropenia, but not with anemia? As adult erythropoiesis is significantly regulated by erythropoietin production in the kidneys, this distinct homeostatic mechanism might account for the independence of anemia from MPL expression in AML patients. Finally, how can we reconcile the TPO-scavenging model of AML cytopenias with existing data showing that AML cells inhibit normal HSC proliferation and differentiation and alter the bone marrow microenvironment?2-4
The biology of TPO and MPL is complex, and TPO and its receptor MPL play pleiotropic and far more important roles in regulating hematopoiesis than simply triggering thrombopoiesis. TPO can both induce expansion of HSCs but also paradoxically maintain HSC quiescence, possibly by operating at different signaling levels.8,9 Clinically, the TPO mimetic eltrombopag promotes trilineage hematopoiesis in patients with aplastic anemia.10 It is therefore possible that loss of TPO in AML patients might partially explain the inhibition of normal HSC and progenitor cell proliferation and differentiation. If MPL-mediated TPO scavenging is indeed a major driver of anemia and neutropenia in AML patients, could additional TPO rescue normal HSCs? Might TPO itself inhibit expression of the HSC quiescence-inducing transcription factor Egr3?3 These concepts provide fertile ground for laboratory experiments. However, given the potentially growth-promoting properties of TPO for AML blasts,6,7 caution is warranted when considering the use of TPO in AML patients with cytopenias. As an alternative, blast eradication with induction chemotherapy serves as an effective method for increasing TPO levels in AML patients with MPL-expressing blasts.6
Although it is unlikely that TPO scavenging by MPL is the only determinant of cytopenias in AML patients, the work by Rauch et al supports an intriguing and novel model to explain the impairment of normal hematopoiesis in patients with AML. By stealing TPO, high levels of MPL on AML blasts might leave AML patients down for the count.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal