In this issue of Blood, Podoplelova et al reveal how structural features of procoagulant platelets, generated in response to strong agonists, eg, thrombin and collagen-related peptides, provide an extensively folded surface area for the membrane-dependent assembly of tenase and prothrombinase complexes and subsequent thrombin generation.1 In brief, the structural features of a small, specialized region of the surface of procoagulant platelets, termed the “cap,” likely contribute strongly to localized amplification of coagulation in thrombosis.
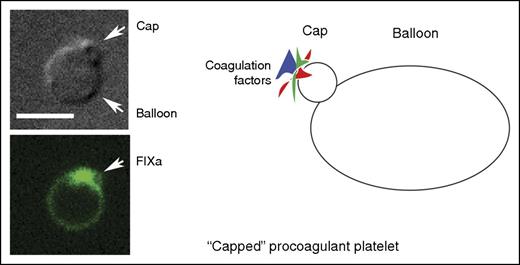
A “capped” procoagulant platelet as shown by differential interference contrast microscopy, fluorescence microscopy (green, factor IXa labeling), and in cartoon form. Scale bar, 5 μm. Micrographs are from Figure 2A in the article by Podoplelova et al. See the complete figure in the article by Podoplelova et al that begins on page 1745.
A “capped” procoagulant platelet as shown by differential interference contrast microscopy, fluorescence microscopy (green, factor IXa labeling), and in cartoon form. Scale bar, 5 μm. Micrographs are from Figure 2A in the article by Podoplelova et al. See the complete figure in the article by Podoplelova et al that begins on page 1745.
Capping and cap formation is normally associated with nucleated cells and was first described in 1971 by Taylor et al for lymphocyte surface immunoglobulin molecules.2 It is an induced process, in this case by anti-immunoglobulin antibody, in which the multivalent ligand selectively aggregates “receptors” into patches that generate a cell surface protein cluster, the cap. The clustered protein cap can later be internalized into the cell interior. In contrast, the protein cluster, or cap, of the procoagulant platelet is formed but not internalized. Rather, what is generated is an assembly of blood coagulation proteins sequestered on the exterior surface of a set of membrane infoldings at 1 pole of the procoagulaant platelet (see figure). Given the platelet's propensity for endocytosis, these infoldings could represent abortive pinocytic events. This may be a consequence of the “zombie-like” nature of procoagulant platelets. Cumulative evidence over the last decade points to procoagulant platelets as being necrotic cells containing a diluted cytoplasm and disrupted cytoskeleton that is nearly devoid of organelles.3-5 Consequently, they adhere poorly to different surfaces.3 Procoagulant platelets are marked by phosphatidylserine (PS) exposed by an agonist induced anionic phospholipid inversion at the plasma membrane. The exposed PS and its localization can be readily revealed by the PS-sensitive marker annexin V. Surface PS facilitates assembly of the tenase and prothrombinase complexes.1,6
As observed by Podoplelova et al and others,4 the procoagulant platelets formed on strong stimulation resembled large balloons with an ∼1-μm radius cap-like region enriched in adhesive proteins. Although the balloon formation may be due to the degradation of the platelet cytoskeleton, how does the cap form? The movement of coagulant factors into the cap, unlike protein concentration in nucleated cells, could be a blood flow dependent process rather than an active platelet process. Experimentally, the importance of shear to cap formation was demonstrated in a collagen-dependent arterial model and the surface complexity of the induced caps was shown by electron microscopy. Some coagulation factor binding on the balloon was found, but the actual concentrations were much lower than in the cap after considering protein amounts and membrane surface area (see figure). The use of model PS-positive liposomes and mathematical modeling demonstrated quantitatively that surface binding increased coagulation-dependent reactions.
In sum, a significant outcome of this study is the suggestion that much of the function of the zombie procoagulant platelet is to provide a specialized membrane surface for the organization of coagulant factors and putative protection of the bound factors from shear forces and dilution due to the infoldings of the cap. As suggested by the authors, whether this occurs in vivo in arterioles will require future experimentation and intravital microscopy. The relationship of procoagulant platelets to the platelet plug as described in a spatially defined wound model7 remains an open question. Whether the process requires an active cytoplasm or is a consequence of fluid forces alone can be probed by the use of actin and microtubule inhibitors. Why the cap is not internalized as in other cells is an open point that likely requires a much more detailed understanding of platelet endocytosis and cytoskeleton than presently. Indeed, important questions remain to be answered.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal