In this issue of Blood, Theocharides et al report one of likely many functionally important untoward consequences of mutant calreticulin that may arise in the clinical context of myelofibrosis.1 Even when viewed through the narrow prism of neutrophil biology, mutant calreticulin will adversely affect myeloid cells throughout their lifespan, from the production of critical glycoproteins in bone marrow precursors to the clearance of spent neutrophils.
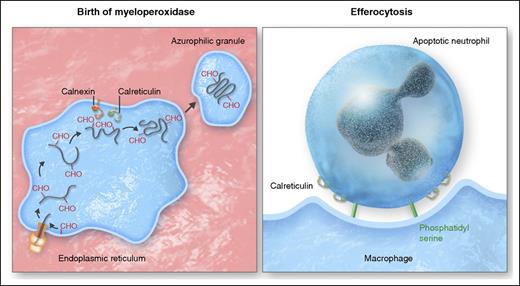
Calreticulin at the birth and death of neutrophils. (Left panel) As molecular chaperones in the endoplasmic reticulum, calnexin and calreticulin play a critical role in the productive biosynthesis of myeloperoxidase and other functionally important glycoproteins generated in neutrophil precursors in the bone marrow, with calreticulin tethered in the ER by binding to the KDEL receptor (yellow). (Right panel) At the end of its life, neutrophils rely again on calreticulin bound to the “eat me” signal phosphatidyl serine to promote efferocytosis of apoptotic neutrophils by macrophages. Professional illustration by Somersault18:24.
Calreticulin at the birth and death of neutrophils. (Left panel) As molecular chaperones in the endoplasmic reticulum, calnexin and calreticulin play a critical role in the productive biosynthesis of myeloperoxidase and other functionally important glycoproteins generated in neutrophil precursors in the bone marrow, with calreticulin tethered in the ER by binding to the KDEL receptor (yellow). (Right panel) At the end of its life, neutrophils rely again on calreticulin bound to the “eat me” signal phosphatidyl serine to promote efferocytosis of apoptotic neutrophils by macrophages. Professional illustration by Somersault18:24.
Calreticulin predominantly resides in the endoplasmic reticulum (ER), where it operates with calnexin and several other ER-resident proteins as part of the quality control system that oversees production of nascent N-linked glycoproteins.2 Like chaperones assigned in social settings to discourage premature and unwarranted interactions between individuals, calreticulin and calnexin associate transiently with biosynthetic intermediates to facilitate their proper and productive maturation, but are not part of the final product (see figure). One of the earliest demonstrations that calreticulin was a bona fide ER chaperone for endogenous glycoproteins arose from studies of the biosynthesis of myeloperoxidase (MPO), the hemoprotein localized exclusively in the granules of neutrophils and monocytes.3 Normal MPO precursors in the ER interact transiently with calreticulin and calnexin,4 and mutant forms of MPO that cause inherited MPO deficiency exhibit aberrant interactions with ER chaperones.5
Although calnexin and calreticulin share many functional attributes, calnexin is an integral membrane protein, whereas calreticulin resides as a soluble protein in the ER lumen, targeted there by binding of its carboxy terminal 4 amino acids, lysine-aspartic acid-glutamic acid-leucine (KDEL), to a KDEL receptor (see figure). Just as targeted interruption of the KDEL sequence of calreticulin in experimental systems disrupts its normal location and function,6 mutation of the KDEL sequence of calreticulin in myeloid precursors of 5 patients reported by Theocharides et al undermines productive processing and packaging of MPO, despite normal expression of the MPO gene. However, given the central role of calreticulin broadly in glycoprotein synthesis, many clinically important N-linked glycoproteins in addition to MPO, such as eosinophil peroxidase observed in this report, likely fail to mature or localize optimally in these patients.
Defective calreticulin exacts a clinical toll in these patients with myelofibrosis. In normal neutrophils, MPO catalyzes the generation of the potent microbicide hypochlorous acid in phagosomes, thereby creating an environment toxic and often lethal to ingested microbes.3 Consequently, one would predict that neutrophils unable to support MPO-dependent biochemistry would leave the host susceptible to frequent and severe infections, akin to the clinical predicament of patients with chronic granulomatous disease, whose phagocytes have defective reduced nicotinamide adenine dinucleotide phosphate oxidase activity and thus cannot generate oxidants.7 However, individuals with inherited MPO deficiency kill ingested bacteria such as staphylococci and streptococci, but do so more slowly than do normal neutrophils.8 In contrast to the clinical experience of those with inherited MPO deficiency, one third of the patients with acquired MPO deficiency reported by Theocharides et al had severe infections, although not those typical for patients with inadequate neutrophil number or function. The basis for the higher prevalence of infection in these patients vs in patients with inherited MPO deficiency is unknown, but an unexplained observation published nearly half a century ago may provide a subtle clue. Klebanoff reported that the microbicidal activity of normal neutrophils treated with the peroxidase inhibitor sodium azide, which blocks MPO activity, is significantly more impaired than that of neutrophils from MPO-deficient individuals, as if the latter had developed or enlisted antimicrobial activities as partial compensation for the genetic absence of MPO. The lack of such adaptation in acquired MPO deficiency may explain, in part, the greater morbidity from infection in the 2 patients with acquired MPO deficiency reported here. Of course, defective biosynthesis of other host defense glycoproteins likely would also contribute to greater susceptibility to infection. It is noteworthy that the patient with myelofibrosis with incidental inherited MPO deficiency did not have infectious complications.
Calreticulin figures not only in the birth of glycoproteins in myeloid precursors but also in the clearance of dead or apoptotic neutrophils (see figure). An effective inflammatory response must culminate in restoration of tissue homeostasis, which relies on efferocytosis, the active engagement of tissue macrophages to bind, ingest, and clear spent neutrophils from the site, thereby eliminating agents that would otherwise sustain inflammation chronically. Initiation of efferocytosis reflects the balance of competing “eat me” and “don’t eat me” signals from neutrophils that prevents ingestion of viable cells but promotes clearance of apoptotic cells by local macrophages. Phosphatidyl serine and other cell surface elements on apoptotic bodies bind to receptors on tissue macrophages through a complex and incompletely understood series of signaling events to drive their ingestion. Calreticulin associates with the “eat me” signal phosphatidyl serine on the cell surface of apoptotic, but not viable, cells to promote internalization.9 Although the mechanism by which calreticulin redistributes from ER to the plasma membrane is not known, it is plausible that KDEL-deficient calreticulin, as identified by Theocharides et al, would fail to reach the cell surface, thereby depriving the apoptotic neutrophil one of the determinants that drive its removal by macrophages, and leaving the uningested cell to undergo necrosis and promote inflammation. Such a scenario occurs in the setting of cyclin-dependent kinase deficiency, whereby apoptotic smooth muscle cells lack surface calreticulin and resist efferocytosis by local macrophages.10 Uncleared, the residual smooth muscle cells undergo necrosis and foment prolonged inflammation. If true in the context of myelofibrosis with mutated calreticulin, failed efferocytosis of apoptotic cells would compound the other defects that compromise effective host defenses in affected patients.
Taken together, the observations of Theocharides et al set the stage for further exploration of how mutant calreticulin, both as a participant in quality control of nascent glycoprotein synthesis in the ER and as a ligand for efferocytosis of apoptotic cells, figures in the full lifespan of cells, from the birth of glycoproteins to the delivery of dead cells to their final resting place.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal