Key Points
We report a method to optogenetically control the release of soluble mediators, such as chemokines, and influence immune cell migration.
This approach is applicable to a variety of secreted ligands and can facilitate dynamic, in situ studies of immune cell communication.
Abstract
Light-mediated release of signaling ligands, such as chemoattractants, growth factors, and cytokines is an attractive strategy for investigation and therapeutic targeting of leukocyte communication and immune responses. We introduce a versatile optogenetic method to control ligand secretion, combining UV-conditioned endoplasmic reticulum-to-Golgi trafficking and a furin-processing step. As proof of principle, we achieved light-triggered chemokine secretion and demonstrated that a brief pulse of chemokine release can mediate a rapid flux of leukocyte contacts with target cells in vitro and in vivo. This approach opens new possibilities for dynamic investigation of leukocyte communication in vivo and may confer the potential to control the local release of soluble mediators in the context of immune cell therapies.
Introduction
The secretion and graded distribution of diffusible mediators represents a major mode of cell–cell communication that is essential for immune responses. However, we still have a poor understanding of how soluble signaling mediators propagate and act in vivo. What is their temporal and spatial range of action? How much ligand needs to be produced and by how many cells for a biological effect in vivo? How do leukocytes respond to dynamic signal inputs in tissue? The ability to spatiotemporally manipulate production of signaling ligands in situ would provide a means to answer several fundamental questions of this nature and to locally control immune cell functions during cell therapy, yet has so far remained a technological challenge.
One experimental development in this direction is the chemical synthesis of caged signaling ligands.1 For example, administration of chemically produced caged chemokines was shown to control cell positioning of leukocytes in vivo.1 A second approach, by means of optogenetics, is to generate chimeras of G protein–coupled receptors, such as chemokine receptors, with rhodopsin, which has allowed manipulation of leukocyte migration by light.2 However, these approaches bypass natural ligand propagation or recognition. In addition, rhodopsin chimeras are not applicable to non-G protein–coupled receptor signaling. Light-mediated control of protein activity and distribution by means of other optogenetic modules, such as CRY2, LOV, or UVR8, offer further possibilities to manipulate cell signaling.3 Optogenetic control of ligand release in vivo would represent a very attractive strategy to interrogate basic mechanisms of intercellular communication by diffusible proteins. Such an approach would offer new possibilities over existing methods: (1) use of a cellular source for production of the soluble mediator, allowing dynamic studies of intercellular communication; (2) targeted expression in specific cell types or tissues; (3) minimized intervention with physiological ligand propagation and recognition mechanisms; and (4) absence of added exogenous factors, a potential a source of complication for in vivo studies and applications. Although strategies for transcriptional control of ligand production have been described (ranging from optogenetically controlled,4,5 pharmacologically controlled,6 or temperature-controlled transcription7 ), such approaches have limited dynamic range because ligand would require a considerable amount of time to be secreted by the cells. Strategies to trigger ligand secretion are more rapid, but have so far been based on chemical–genetic induction.8,9
Here we present a first optogenetic method to deliver rapid pulsatile ligand secretion and manipulate cell communication by light in situ.
Methods
Animals
C57BL/6 transgenic mice expressing green fluorescent protein (GFP) under the control of the human ubiquitin C promoter10 in wild-type or Rag2−/− background were used for the transwell chemotaxis experiments. BALB/c LysM-enhanced GFP (EGFP)11 mice were used for in vivo interaction assays and mTomato mice12 were used for in vitro interaction assays. All mice were bred under specific pathogen-free conditions at Institut Pasteur. Mouse experiments were performed in accordance with the guidelines of Institut Pasteur for animal care and use. Tg(mpx:Lifeact-Ruby) and Tg(lyz:DsRed)nz50 zebrafish were bred and handled in the University of Cambridge according to institutional and UK guidelines (Home Office License number: 70/8255).
Plasmids
sec-mCherry (as in Sarris et al13 ), murine Cxcl2, or zebrafish Cxcl8 were designed to be at the N-terminal end, followed by a linker sequence, a furin cleavage site (amino acid sequence SARNRQKR), VSVG-ts045, yellow fluorescent protein (YFP), and 2 UVR8 repeats. Inserts were synthesized by GeneWiz and cloned using BamHI and Hind III into a retroviral and mammalian expression vector pMSCV. Wild-type Cxcl2 was synthesized alongside and cloned using BamHI and Hind III into pMSCV. Zebrafish Cxcl8 was subcloned into the same backbone vector pMSCV-YFP-2xUVR8.
Cell culture and transfection
HEK293T cells were cultured in Dulbecco’s modified Eagle medium (Invitrogen) containing 10% fetal bovine serum (Hyclone) and were transfected with plasmids using Lipofectamine 2000 (Invitrogen). With the exception of zebrafish cell interaction experiments, HEK293T cells were used 16 to 24 hours posttransfection.
Imaging of mCherry-UVR8 redistribution
HEK293T cells were transfected in imaging dishes (Ibidi); 16 to 18 hours later, the cells were exposed to UVB light (3-24 mJ) using an X97 UVB-BB (280-320 nm) lamp (DAAVLIN). Immediately after, the cells were imaged in an Olympus Confocal System (FV1000MPE) using a 40×/0.95 numerical aperture (NA) objective or a Leica SP8 confocal using a 20×/0.75 NA objective. mCherry was excited using a 560-nm laser and YFP was excited using a 488-nm laser. Thin z-stacks of about 10-µm thickness with a spacing of approximately 2 µm were acquired. Maximum intensity projection of confocal stacks was performed using ImageJ.
Western blotting
HEK293T cells were transfected and 16 to 18 hours later; medium was replaced with fresh medium. Immediately after, cells were exposed to UVB light (6-24 mJ) using an X97 UVB-BB (280-320 nm) lamp, and then incubated for 2 to 3 hours at 32°C. Conditioned medium was then concentrated 50- to 100-fold using Vivaspin columns (Sartorius) of appropriate molecular weight cutoff (as an indication, a blot lane would typically be loaded with supernatant corresponding to one-fourth of a confluent 10-cm dish of HEK293T cells). Cell lysates were prepared in RIPA buffer (Invitrogen) and EDTA-free protease inhibitors (Roche). Samples were run on a 10% to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Nupage) and blotted using the Nupage iBlot system. For detection of mCherry, anti-DsRed (Clontech) was used at 1:1000; for detection of Cxcl2, we used anti-Cxcl2 (R&D Systems) at a dilution of 1:300 to 1:1000. Revelation was performed using a Supersignal West Femto Maximum Sensitivity Substrate kit (Life Technologies) or ECL Prime Western Blotting Detection kit (GE Healthcare). For the time course of Cxcl2 secretion, we collected cell supernatants in 30-minute intervals after photoactivation, washing the cells between collection time points, to assess new chemokine secretion. For this experiment, 30-second exposure to a UVB source of wavelength 312 nm was used (EB280C supported by a SE-140 stage; UVMAN). For calibration of protein amount, recombinant Cxcl2 (R&D Systems) of known concentration was loaded on the blot. Lane densitometry was performed in Fiji.
In vitro transwell chemotaxis assay
HEK293T cells 1 day posttransfection with UVR8-YFP constructs were briefly washed, without disturbing the cell monolayer, and replenished with fresh medium (serum-free and phenol red-free RPMI). Cells were exposed to UVB (9 mJ), using an X97 UVB-BB (280-320 nm) lamp, and incubated at 32°C for 3 to 4 hours. Supernatants were concentrated (about 12-fold) and stored at −20°C for use the following day. GFP+ neutrophils were prepared from bone marrow extracted from Rag−/−xUbiGFP mice, using the Miltenyi Neutrophil isolation kit (Miltenyi, negative selection kit). Single cell suspensions were made in RPMI/0.5% bovine serum albumin (Calbiochem)/10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Invitrogen) at a density of 1 to 1.3 × 106 cells/mL. Chemotaxis was assessed in a 96-well HTS Transwell system (Corning). A total of 240 μL of culture supernatant or RPMI medium was added to the bottom chamber (each well would typically be loaded with supernatant corresponding to one-half of a 10-cm dish of HEK293T cells). In some cases, supernatants were preincubated in anti-Cxcl2 (2 µg/mL final concentration; R&D Systems) for 15 to 30 minutes on ice and then placed in the bottom chamber. A total of 75 μL of neutrophil suspensions was placed in the upper chamber. After incubation for 3 hours at 37°C, cells in the bottom chamber were harvested and the number of GFP+ cells was assessed by fluorescence-activated cell sorting (FACS) analysis (FACS Canto, BD). The results were normalized to Calibrite beads (BD) that had been added to each cell sample just before harvest, to ensure independence from volume and FACS fluctuations.
In vitro cell interaction assay
HEK293T cells were seeded in 35-mm imaging dishes (Ibidi) coated with poly-l-lysine (Sigma) and transfected in these dishes 1 day postseeding. Neutrophils were purified from bone marrow extracted from mTomato mice12 using the Miltenyi Neutrophil isolation kit (Miltenyi, negative selection kit). Purity of the cell preparation was assessed via FACS and staining for the markers Ly6G and CD11b. Fresh neutrophils were resuspended in imaging medium (RPMI phenol red-free/10% fetal bovine serum/10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and seeded on to HEK293T cells that had been transfected the previous day in imaging plates. Neutrophils were allowed to settle for a few minutes and then the culture was exposed to UVB light (doses ranging from 6 to 24 mJ), using an X97 UVB-BB (280-320 nm) lamp. As soon as possible after UV exposure, plates were imaged under epifluorescence using a DMI 6000B inverted microscope (Leica Microsystems) equipped with an environmental chamber for temperature, humidity, and 5% CO2 (PECON), with a ×20/0.75 NA (Olympus) or ×10/0.45 NA (Nikon) dry objectives and a CoolSNAP HQ2 Roper camera (Photometrics, Tucson, AZ). Time-lapse movies with an interval of 2 minutes were acquired in a multiplex fashion. Temperature was maintained at 34°C to 35°C. Movies were processed in Imaris (Bitplane) and ImageJ.
Cell interaction assay in mouse tissue
BALB/c LysM-EGFP mice were anesthetized, and the dorsal dermis of the ear was surgically exposed. Transfected HEK293T cells were seeded on the exposed dermis during 1 hour. The ear was then rinsed several times with phosphate-buffered saline and prepared for microscopy. A quartz coverslip (Ted Pella Inc) was placed onto the ear and covered with deionized water to immerge a ×25/1.05 NA objective (Olympus). Two-photon imaging was performed using a DM6000 upright microscope equipped with an SP5 confocal head (Leica Microsystems, Wetzlar, Germany) and a Chamaeleon Ultra Ti:Sapphire laser (Coherent, Santa Clara, CA) tuned at 920 nm. Emitted fluorescence was passed to nondescanned detectors through dichroic mirrors. Typically, images from 15 to 20 z planes spaced at 5 μm were collected every 3 minutes. For UVR8 photoactivation, the ear dermis was exposed for 120 seconds to a UVB source (310 nm LED, Thorlabs) from a distance of 3 cm.
Cell interaction assay in zebrafish
HEK293T cells were transfected with a mock plasmid (pCAG-GFP) or Cxcl8-YFP-2xUVR8 plasmid and 5 hours later were implanted into Tg(mpx:Lifeact-Ruby)14 or Tg(lyz:DsRed)nz50 transgenic zebrafish embryos at 48 hpf as previously described.13 Larvae were kept overnight at 34°C in E3 medium (5 mM NaCl, 0.15 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, methylene blue, and 1-phenyl-2-thiouria). Eighteen to 24 hours posttransplantation, larvae were mounted in 1% low-melting-point agarose in 35-mm dishes topped with E3 medium (without methylene blue) supplemented with 0.16 mg/mL tricaine (Sigma). Time-lapse confocal stacks (of between 35 and 50 slices spaced by 2 µm) were acquired with an interval of 15 to 30 seconds on an upright Nikon E1000 microscope using ×40/0.80 NA water objective coupled to a Yokogawa CSU10 spinning disc confocal scanner unit and illuminated using a Spectral Applied Research LMM5 laser module (491 nm for YFP excitation; 561 nm for Ruby/DsRed) or on a Perkin Elmer Spinning Disk UltraVIEW ERS, Olympus IX81 inverted spinning disk confocal microscope using a ×20/0.45 NA oil objective, 488 nm for YFP excitation, and 561 nm for Ruby/DsRed. Images were captured using Volocity software. Imaging was conducted within a custom-built heated chamber (for the upright scope) or a heated stage (for the inverted scope) and temperature was maintained at 32°C to 35°C. For UVR8 photoactivation, the mounted larvae were exposed for 60 seconds to a UVB source (312 nm; EB280C supported by a SE-140 stage; UVMAN) from a distance of 7 cm (power equivalent to 0.3 mW/cm2 according to Chen et al15 ). When using an inverted scope, embryos were mounted on a quartz coverslip for maximum UV transmission. When using an upright scope, UV illumination was performed directly above the agarose-embedded embryos. Cell tracking analysis was performed using Imaris 8.2 (Bitplane).
Statistics
All error bars indicate standard error of the mean. All P values were calculated with 2-tailed statistical tests and 95% confidence intervals. Statistical tests were performed in Prism5 (GraphPad).
Results and discussion
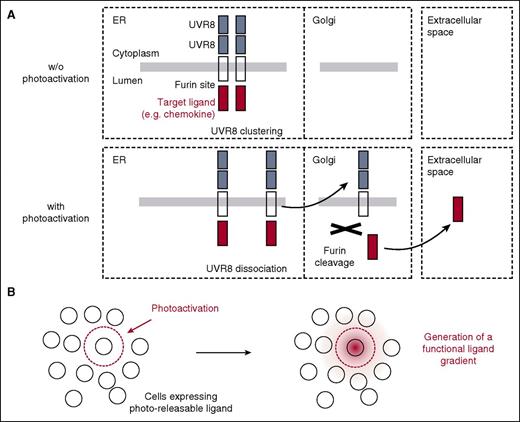
A recent study showed that fusions of VSVG, a model membrane protein, with tandems of UVR8 spontaneously form clusters in the endoplasmic reticulum (ER) that are unable to traffic to the Golgi.15 Upon UV exposure, some of the clustered molecules are released and traffic to the plasma membrane within minutes.15 This is evident immediately after activation as a more diffuse and extensive intracellular distribution.15 It remains unclear whether UVR8 can be used for control of secreted proteins and for functional studies of any kind. We thus sought to establish a strategy tailored to signaling effectors secreted in the extracellular milieu by exploiting the light-responsive properties of UVR8 domains. We reasoned that a simple fusion of a secreted effector ligand with UVR8, as applied so far for control of membrane protein traffic,15 might encounter at least 3 complications: (1) photoactivation of UVR8 might not be as effective in the ER lumen as in the cytoplasm, which is the natural site of UVR8 activation16 ; (2) the large size of UVR8 could potentially interfere with biological activity and extracellular diffusion and presentation; and (3) the possible reoligomerization of UVR8 in the extracellular environment cannot be easily measured and excluded in vivo. To prevent these complications, we exploited the fact that many secreted effectors, including hormones and growth factors, are naturally processed into their active form by furin, a Golgi-resident enzyme.17 We engineered constructs so that the target, secreted protein is fused to the N-terminal, luminal part of a type I membrane protein (VSVG-ts045) with a consensus furin recognition sequence between the target secreted protein and the “carrier” transmembrane protein (Figure 1). The C-terminal, cytoplasmic part of this construct contains 2 UVR8 tandems (Figure 1). Because furin is resident in the Golgi,17 molecules trafficking to the Golgi after photoactivation should be cleaved, leading to photo-dependent release of the target protein in the extracellular medium without the UVR8 moiety (Figure 1). Thus, this strategy would maintain UVR8 in the cytoplasm and prevent any effects of UVR8 on extracellular protein activity and presentation, ensuring applicability to versatile ligands without special customizations.
Strategy for optogenetic control of ligand secretion. (A) Strategy for optogenetic triggering of ligand secretion. The target ligand is fused to the luminal side of a transmembrane protein (VSVG-ts045) and YFP and 2xUVR8 repeats on the cytoplasmic side. Before photoactivation (no PA), the construct forms clusters in the ER, with minimal trafficking to the Golgi. After photoactivation by UVB (PA), some fusion proteins get released and traffic to the Golgi whereby the target ligand is cleaved off by furin, leading to secretion of the desirable moiety in the extracellular medium. (B) Representation of cells in tissue before and after photoactivation. Target ligand gradient is shown in red. w/o, without.
Strategy for optogenetic control of ligand secretion. (A) Strategy for optogenetic triggering of ligand secretion. The target ligand is fused to the luminal side of a transmembrane protein (VSVG-ts045) and YFP and 2xUVR8 repeats on the cytoplasmic side. Before photoactivation (no PA), the construct forms clusters in the ER, with minimal trafficking to the Golgi. After photoactivation by UVB (PA), some fusion proteins get released and traffic to the Golgi whereby the target ligand is cleaved off by furin, leading to secretion of the desirable moiety in the extracellular medium. (B) Representation of cells in tissue before and after photoactivation. Target ligand gradient is shown in red. w/o, without.
We first tested this approach in HEK293T cells using a model, secreted mCherry (mCherry with a chemokine signal sequence13 ) as target ligand and YFP as a fluorescent reporter for the UVR8 moiety (see supplemental Figure 1A, available on the Blood Web site). A short pulse of UVB light led to a redistribution of mCherry, along with YFP (supplemental Figure 1B). Redistribution was similar in a construct that did not carry the furin recognition site confirming that there is no obvious leakiness of furin processing that could lead to mCherry mislocalization (supplemental Figure 1B). Importantly, redistribution of mCherry-YFP-2xUVR8 (with the furin recognition site) was accompanied by release of mCherry in HEK293T cell supernatants (supplemental Figure 1C-D). In contrast, mCherry was not detectable in supernatants from cells that were not exposed to UV light or that expressed a construct lacking the furin cleavage site (supplemental Figure 1C-D), confirming the photo-dependent and furin-dependent control of mCherry secretion.
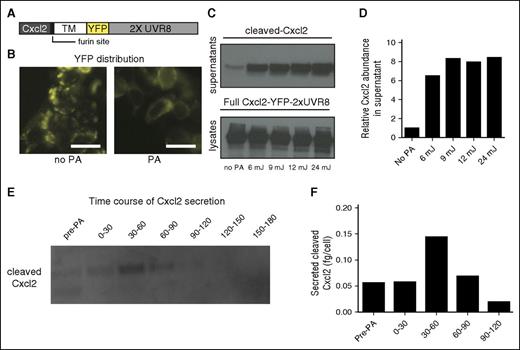
Next we tested this approach with a chemokine. We replaced secreted mCherry with mouse Cxcl2, the murine homolog of human CXCL8 (or interleukin-8), a well-characterized neutrophil attractant (Figure 2A). Although the chemokine is not directly visible, the cytoplasmic YFP can be used as readout for successful photoactivation, a highly useful feature of this system (Figure 2B). Using different doses of UV light, we found that a minimum dose of 6 mJ could lead to significant release of the chemokine in the supernatant (Figure 2C-D). Consistent with previous reports,15 we found that the minimum dose of UV required for UVR8 activation does not cause detectable cell toxicity (supplemental Figure 2). Notably, we detected 2 specific bands for Cxcl2 in western blots of cell culture supernatants (supplemental Figure 1E). In agreement with other reports on chemokine glycosylation,18 we found that the higher molecular weight band corresponded to a glycosylated form of Cxcl2, as the band could be eliminated after PNGase treatment (Figure 2C). We further quantified the kinetics of Cxcl2 secretion. To this end, we collected cell supernatants in 30-minute intervals after photoactivation to assess new chemokine secretion. We found that the majority of chemokine release occurs between 30 and 60 minutes post-UV exposure and is in the order of 0.15 fg/cell (Figure 2 E-F).
Optogenetic control of chemokine secretion. (A) Construct design for optogenetic triggering of murine Cxcl2. (B) Distribution of Cxcl2-YFP-2xUVR8 before or after PA with 9 mJ. Scale bar = 20 µm. (C) Detection of cleaved Cxcl2 in supernatants and uncleaved Cxcl2-YFP-2xUVR8 in lysates from nontreated (no PA) or photo-activated cells with indicated doses of UVB. Supernatant samples were treated with PNGase to obtain single bands representing nonglycosylated chemokine. (D) Quantification of protein in the supernatant samples shown in panel C, based on band intensity, normalized against the lysate bands and relative to the “no PA” condition. (E) Time course of cleaved Cxcl2 secretion. Supernatants were collected in 30-minute intervals, washing the cells between collection time points, to assess new chemokine production. (F) Quantification of exact protein amount shown in panel E. TM, transmembrane protein anchor.
Optogenetic control of chemokine secretion. (A) Construct design for optogenetic triggering of murine Cxcl2. (B) Distribution of Cxcl2-YFP-2xUVR8 before or after PA with 9 mJ. Scale bar = 20 µm. (C) Detection of cleaved Cxcl2 in supernatants and uncleaved Cxcl2-YFP-2xUVR8 in lysates from nontreated (no PA) or photo-activated cells with indicated doses of UVB. Supernatant samples were treated with PNGase to obtain single bands representing nonglycosylated chemokine. (D) Quantification of protein in the supernatant samples shown in panel C, based on band intensity, normalized against the lysate bands and relative to the “no PA” condition. (E) Time course of cleaved Cxcl2 secretion. Supernatants were collected in 30-minute intervals, washing the cells between collection time points, to assess new chemokine production. (F) Quantification of exact protein amount shown in panel E. TM, transmembrane protein anchor.
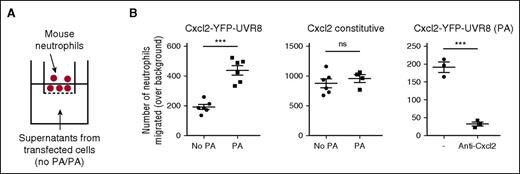
To verify the functionality of the photo-released chemokine, we used concentrated culture supernatants from photo-activated cells in a transwell chemotaxis assay (Figure 3A). Supernatants from photo-activated cells were more attractive to neutrophils than supernatants from nonphotoactivated Cxcl2-YFP-2xUVR8–expressing cells and this attractive potential could be blocked by anti-Cxcl2 blocking antibody, confirming that the photo-released chemokine is functional (Figure 3B). The strong effect shown for the antibody blocking reflects inhibition of both photo-released chemokine and background chemokine (from dead cells or leakiness in secretion) in the concentrated supernatants. Supernatants from cells expressing wild-type, constitutive Cxcl2, were equally attractive to neutrophils with or without UV exposure, confirming that light exposure specifically affects UVR8-conditioned protein trafficking (Figure 3B).
Photo-released chemokine is functional. (A) Evaluation of functionality of photo-released chemokine in a transwell chemotaxis assay. The bottom chamber was loaded with supernatants from cells transfected with a mock plasmid or Cxcl2-YFP-2xUVR8 or constitutive Cxcl2 and then photo-activated or not. Freshly isolated mouse neutrophils were placed in the top chamber. (B) Number of neutrophils transmigrated over background migration (determined by migration to mock supernatants). Data points represent multiple wells within 1 experiment representative of 4 independent experiments. Unpaired t test was applied.
Photo-released chemokine is functional. (A) Evaluation of functionality of photo-released chemokine in a transwell chemotaxis assay. The bottom chamber was loaded with supernatants from cells transfected with a mock plasmid or Cxcl2-YFP-2xUVR8 or constitutive Cxcl2 and then photo-activated or not. Freshly isolated mouse neutrophils were placed in the top chamber. (B) Number of neutrophils transmigrated over background migration (determined by migration to mock supernatants). Data points represent multiple wells within 1 experiment representative of 4 independent experiments. Unpaired t test was applied.
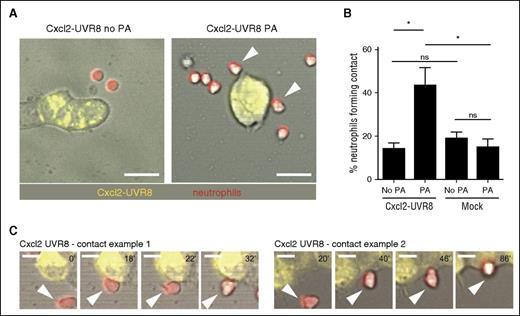
Next, we investigated whether short pulses of chemokine could affect neutrophil behavior. We cocultured HEK293T cells expressing Cxcl2-YFP-2xUVR8 with primary mouse neutrophils and exposed the cells to UV (Figure 4A). We titrated the dose of UV to minimize any potential effects on neutrophil motility. We found that a single pulse of UV was sufficient to substantially increase the frequency of interactions between neutrophils and Cxcl2-YFP-2xUVR8 expressing cells (Figure 4B-C; supplemental Video 1). In contrast, UV exposure did not affect the frequency of interactions with mock-transfected cells (Figure 4B).
A brief, light-triggered pulse of chemokine secretion rapidly enhances cell interactions in vitro. (A) Representative images of cocultured HEK293T cells and mouse neutrophils before and after activation. White arrows indicate contacts. Scale bar = 25 µm. (B) Percentage of neutrophils forming contacts with transfected HEK239T cells (out of the number of neutrophils present in the same area). Repeated measures ANOVA with Bonferroni posttest was applied. n = 3 values representing independent experiments. Error bars represent standard error of the mean. (C) Examples of contact formation after photoactivation of Cxcl2 secretion. Scale bar = 10 µm.
A brief, light-triggered pulse of chemokine secretion rapidly enhances cell interactions in vitro. (A) Representative images of cocultured HEK293T cells and mouse neutrophils before and after activation. White arrows indicate contacts. Scale bar = 25 µm. (B) Percentage of neutrophils forming contacts with transfected HEK239T cells (out of the number of neutrophils present in the same area). Repeated measures ANOVA with Bonferroni posttest was applied. n = 3 values representing independent experiments. Error bars represent standard error of the mean. (C) Examples of contact formation after photoactivation of Cxcl2 secretion. Scale bar = 10 µm.
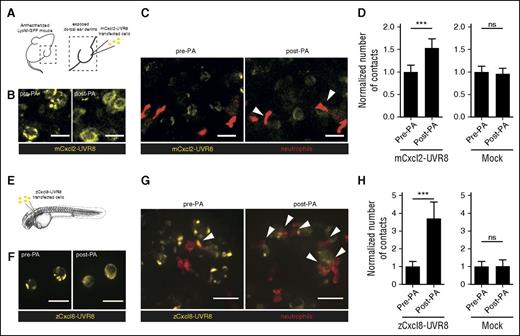
Finally, we evaluated the potential to photo-activate chemokine secretion in a tissue context. We seeded mock or Cxcl2-YFP-2xUVR8-transfected HEK293T cells in surgically exposed ear dermis from BALB/c LysM-EGFP mice,11 in which endogenous neutrophils express GFP (Figure 5A). Consistent with our in vitro evidence, we found that a single pulse of UV light led to an immediate change in Cxcl2-YFP-2xUVR8 distribution from perinuclear aggregates to diffuse intracellular distribution (Figure 5B) and a subsequent (within 30 minutes) increase in the number of contacts between transplanted cells and endogenous neutrophils (Figure 5C-D; supplemental Video 2). A UV light pulse on mock-transfected HEK293T cells did not alter neutrophil behavior, confirming that the effect was dependent on the photo-released chemokine (Figure 5D). To further validate the in vivo applicability of our approach, we performed analogous experiments in zebrafish with the corresponding chemokine homolog (zebrafish Cxcl813 ). We locally injected zCxcl8-YFP-UVR8–transfected cells into Tg(mpx:Lifeact-Ruby) or Tg(lyz:DsRed) zebrafish embryos, in which neutrophils express a red fluorescent actin polymerization probe14 or DsRed, respectively, and imaged neutrophil behavior 1-day posttransplantation (Figure 5E). Exposure of the larvae to a short pulse of UV light led to quick redistribution of zCxcl8-YFP-2xUVR8 (Figure 5F) and a marked increase (within 30 minutes) in the frequency of interactions between neutrophils and transplanted cells and a significant increase in the duration of contacts (Figure 5G-H; supplemental Figure 3A; supplemental Video 3) that was not seen in mock-transfected control experiments (Figure 5H; supplemental Video 4). Motility levels were not grossly affected by the photo-released chemokine; however, we observed a significant increase in the path straightness of the cells, suggestive of more directed migration (supplemental Figure 3B,D). The level of neutrophil accumulation at the site of zCxcl8 release was comparable to that observed with constitutively secreted zCxcl8 by the same type of cellular source,13 suggesting that gradient formation can be established immediately after secretion and does not require large amounts of ligand to be functional. Thus, photoactivation of UVR8 and manipulation of chemokine function can be achieved in an entirely noninvasive manner in organism/tissue samples.
A brief, light-triggered pulse of chemokine secretion rapidly enhances cell interactions in vivo in mouse tissue and in zebrafish. (A) Experimental scheme for optogenetic control of chemokine release in mouse tissue. The ear dermis from an anesthetized LysM-GFP mouse was surgically exposed and seeded with HEK293T cells expressing Cxcl2-YFP-2xUVR8. (B) Distribution of Cxcl2-YFP-2xUVR8 before and after PA. (C) Representative images of ear tissue with mouse neutrophils (red) and HEK293T cells (yellow). Arrows indicate contacts between the cells. (D) Number of contacts formed between individual HEK293T cells and neutrophils before and after PA, normalized to number of neutrophils in the field and relative to no PA condition (n = 63 Cxcl2-YFP-2xUVR8-transfected; n = 48 mock GFP-transfected HEK293T cells, pooled data from 2 to 4 250 × 250 μm regions of interest per mouse and at least 3 mice per experimental condition, paired t test). (E) Experimental scheme for optogenetic control of chemokine release in zebrafish. Mock or zebrafish Cxcl8-YFP-UVR8–transfected HEK293T cells were locally transplanted in transgenic mpx:Lifeact-Ruby zebrafish larvae and mounted for imaging and photoactivation. (F) Distribution of Cxcl8-YFP-UVR8 before and after PA. (G) Representative images of zebrafish neutrophils (red) in the area of HEK293T cell implantation (yellow). (H) Number of contacts formed between individual HEK293T cells and neutrophils before and after PA, normalized to number of neutrophils in the field and relative to no PA condition (n = 43 Cxcl8-YFP-UVR8–transfected and n = 18 mock GFP-transfected HEK293T cells, pooled data from 3 larvae in 2 independent experiments, paired t test). Error bars represent standard error of the mean. Scale bar = 25 µm in all images.
A brief, light-triggered pulse of chemokine secretion rapidly enhances cell interactions in vivo in mouse tissue and in zebrafish. (A) Experimental scheme for optogenetic control of chemokine release in mouse tissue. The ear dermis from an anesthetized LysM-GFP mouse was surgically exposed and seeded with HEK293T cells expressing Cxcl2-YFP-2xUVR8. (B) Distribution of Cxcl2-YFP-2xUVR8 before and after PA. (C) Representative images of ear tissue with mouse neutrophils (red) and HEK293T cells (yellow). Arrows indicate contacts between the cells. (D) Number of contacts formed between individual HEK293T cells and neutrophils before and after PA, normalized to number of neutrophils in the field and relative to no PA condition (n = 63 Cxcl2-YFP-2xUVR8-transfected; n = 48 mock GFP-transfected HEK293T cells, pooled data from 2 to 4 250 × 250 μm regions of interest per mouse and at least 3 mice per experimental condition, paired t test). (E) Experimental scheme for optogenetic control of chemokine release in zebrafish. Mock or zebrafish Cxcl8-YFP-UVR8–transfected HEK293T cells were locally transplanted in transgenic mpx:Lifeact-Ruby zebrafish larvae and mounted for imaging and photoactivation. (F) Distribution of Cxcl8-YFP-UVR8 before and after PA. (G) Representative images of zebrafish neutrophils (red) in the area of HEK293T cell implantation (yellow). (H) Number of contacts formed between individual HEK293T cells and neutrophils before and after PA, normalized to number of neutrophils in the field and relative to no PA condition (n = 43 Cxcl8-YFP-UVR8–transfected and n = 18 mock GFP-transfected HEK293T cells, pooled data from 3 larvae in 2 independent experiments, paired t test). Error bars represent standard error of the mean. Scale bar = 25 µm in all images.
In summary, we present a new strategy to functionally control ligand secretion and cell communication by light in vivo that is widely applicable to a variety of signaling proteins including chemoattractants, cytokines, growth factors, and morphogens. Several studies have shown that chemokines can enhance leukocyte contact dynamics.19-21 We provide evidence that a short pulse of chemokine release is sufficient to alter the behavior of neighboring cells within minutes, suggesting that chemokine propagation and functional presentation can be established very rapidly in vivo. The ability to manipulate release of diffusible, signaling ligands in vivo in a pulsatile fashion should open new avenues for exploring how cells communicate during development, at steady state or in the context of an immune response. Finally, our methodology may offer new possibilities in the context of immune cell therapies by offering spatiotemporal control for the release of desirable soluble effectors.
This article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Matthew Kennedy for donation of the VSVG-2xUVR8 plasmid, Anna Huttenlocher for donation of the Tg(mpx:Lifeact-Ruby) transgenic line, Matthew Albert for access to the X97 UVB-BB (280-320 nm) UVB lamp source, Philippe Herbomel for access to an SP8 confocal microscope, and Hélène Moreau for comments on the manuscript.
This work was supported by Institut Pasteur, INSERM, the European Research Council (starting grant LymphocyteContacts) (P.B.), and the Medical Research Council (RG73189) (M.S.).
Authorship
Contribution: M.S. conceived the strategy. M.S., R.O., and P.B. designed the research. M.S. and R.O. performed experiments and analyzed the data. M.S. and P.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Milka Sarris, Department of Physiology, Development and Neuroscience, University of Cambridge, Downing St, Cambridge, CB2 3DY, United Kingdom; e-mail: ms543@cam.ac.uk; and Philippe Bousso, Dynamics of Immune Responses Unit, Institut Pasteur, 75015 Paris, France; e-mail: philippe.bousso@pasteur.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal