In this issue of Blood, Hubbard et al provide important proof-of-concept data on the utility of using genome editing to knock-in a wild-type (WT) complementary DNA (cDNA) to functionally correct disease-causing mutations in a gene in primary human T cells.1
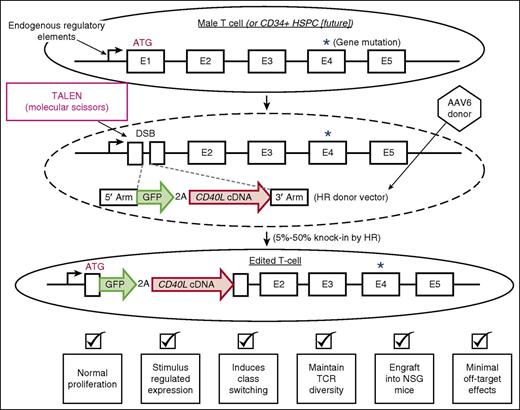
Genome editing to perform cDNA knock-in as a functional gene correction strategy. The figure schematizes the overall strategy and key results of using genome editing to knock-in a WT cDNA to functionally correct downstream mutations. The dashed oval depicts that the TALEN mRNA was delivered into the T cells by electroporation. The dashed gray lines depict HR between the endogenous gene containing a nuclease-induced DSB and the donor vector. 3′ arm, 3′ homology arm; 5′ arm, 5′ homology arm; ATG, endogenous initiation codon; E1-E5, exons 1-5; TCR, T-cell receptor.
Genome editing to perform cDNA knock-in as a functional gene correction strategy. The figure schematizes the overall strategy and key results of using genome editing to knock-in a WT cDNA to functionally correct downstream mutations. The dashed oval depicts that the TALEN mRNA was delivered into the T cells by electroporation. The dashed gray lines depict HR between the endogenous gene containing a nuclease-induced DSB and the donor vector. 3′ arm, 3′ homology arm; 5′ arm, 5′ homology arm; ATG, endogenous initiation codon; E1-E5, exons 1-5; TCR, T-cell receptor.
Although genome editing to correct individual disease-causing mutations is conceptually simple, this approach is currently not feasible for logistical and regulatory reasons because for most monogenic diseases the causative mutations are spread throughout the gene. It would be more practical, therefore, to develop a safe and effective one-size-fits-all approach. Hubbard et al built on the prior work of others to develop such a strategy.2
Although gene-therapy clinical trials using next-generation lentiviral and self-inactivating retroviral vectors are showing safety and exciting efficacy, the long-term risk of insertional oncogenesis is still unknown.3,4 For certain genes, effective and safe therapy requires precisely regulated expression. An example is CD40L, mutations in which are the most common cause of X-linked hyper-immunoglobulin M (X-HIGM) syndrome. Although unregulated expression of CD40 ligand (CD40L) has been shown to rescue CD40L deficiency, the persistent expression also caused a malignant lymphoproliferation after lentiviral delivery.5 Thus, CD40L is an interesting candidate to develop therapeutic genome editing.
In genome editing, a DNA double-strand break (DSB) is created at a specific site in the genome using an engineered nuclease.6 This DSB activates the cell’s repair machinery. If the break is repaired by nonhomologous end joining, small insertions/deletions can be created at the break inactivating a genetic element. If the break is repaired by homologous recombination (HR) using a donor molecule with long homology arms, then defined nucleotide changes can be created in the genome. Hubbard et al used HR to precisely insert a multigene cassette.1,6 In primary T cells and hematopoietic stem and progenitor cells (HSPCs), the introduction of the nuclease or donor as naked DNA causes intolerable toxicity. A solution is to deliver the nuclease as messenger RNA (mRNA) or a ribonucleoprotein complex via electroporation and the donor as recombinant adeno-associated virus 6 (rAAV6). Using this system, targeted integration frequencies of 10% to 50% can be achieved in human T cells and HSPCs.7,8
Hubbard et al combined the use of transcription activator-like effector nucleases (TALENs) with AAV6 to generate HR-mediated genome editing of the CD40L gene in T cells derived from both WT donors and patients with CD40L deficiency.1 They designed the AAV6 donor to knock-in a CD40L cDNA such that the cDNA would be expressed utilizing the natural endogenous regulatory elements (see figure). The knock-in cDNA would functionally correct downstream disease-causing mutations. A key feature of the knock-in vector is the use of the redundant codon usage system to create a cDNA that codes for the same protein but is diverged at the nucleotide level. This feature prevents the HR machinery from causing premature recombination before the entire gene cassette is integrated. They used a 2A peptide linkage to introduce a green fluorescent protein (GFP) gene to measure the efficiency of the process and to evaluate whether the knock-in gene cassette was being regulated properly.
The results are impressive. They were able to: (1) generate high efficiencies of targeted knock-in in both WT and patient-derived T cells (5%-50%); (2) engineer cells that regulated the transgene cassette identically to the unedited CD40L gene; (3) show the edited cells (both WT and patient derived) were functionally equivalent to WT cells in vitro in inducing class switching in cocultured B cells and in vivo after transplantation into NSG mice; and (4) show there were minimal off-target effects by functional assays and sequencing of potential off-target sites. These results provide strong data on a cDNA knock-in strategy for genome editing to functionally correct a human disease-associated gene.
Although the clustered regularly-interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) system has achieved notoriety, other nuclease platforms, including TALENs (used, for example, in this work by Hubbard et al), also have great translational potential. The CD40L TALENs were designed to make a DSB close to the endogenous start codon. The authors were also able to achieve high frequencies of HR-mediated editing without having to use small molecules that may increase genomic instability by altering the natural mechanism of DSB repair. They avoided small molecules by assuring they had an efficient, nontoxic method of delivering sufficient donor molecules to each cell.9 The work also demonstrated the importance of designing the donor vector carefully. Although not quantifying the frequency, they found unexpected recombination events between the 3′ untranslated region (UTR) in their donor vector and the endogenous 3′ UTR in the gene, causing an insertion of the transgene cassette while deleting the rest of the gene. In these studies, the gene deletion seemed to have no functional effects but if the region contained important regulatory elements, there might be deleterious consequences.
Although the results are compelling, several outstanding issues remain. The recombination frequencies were impressive but they came by using relatively high multiplicities of infection (MOIs). Lowering the MOI has important translational implications. Perhaps by simply making higher quality vector, lower MOIs will be needed. There could also be other solutions to lowering the MOI. The authors show that the edited cells maintain an equivalently diverse T-cell repertoire to unedited cells, it is unlikely, however, that a T-cell product will be sufficient for long-term disease suppression. For this reason and the expression of CD40L on other types of immune cells, the authors highlight that transferring functional gene correction to editing HSPCs will be important for the long-term cure of patients. The frequency of editing in the patient-derived cells was lower than in WT primary T cells. Determining the reason for this decrease will be important in creating an efficient cell-manufacturing process. Although GFP was useful experimentally, GFP will need to be replaced by a clinically compatible marker to identify and select for edited cells. Given the high frequencies achieved, it is also possible a marker might not be needed. Finally, although the overall approach has a favorable toxicity profile, a more thorough evaluation of the overall manufacturing process, not just of nuclease specificity, is needed before moving to the clinic.10
In sum, this compelling strategy has great implications for patients with X-HIGM syndrome and provides a blueprint for how other genetic diseases might be cured using genome editing.
Conflict-of-interest disclosure: M.H.P. is a scientific founder with equity and consults for CRISPR Therapeutics. The company had no input into the content of this commentary.