Key Points
Fas-mutated B cells accumulate in the memory compartment and are highly mutated and polyreactive.
Fas deficiency leads to an intrinsic defect in B cells selection that predisposes to autoimmunity and identifies B cells as therapeutic targets for autoimmune lymphoproliferative syndrome.
Abstract
Fas is a transmembrane receptor involved in the maintenance of tolerance and immune homeostasis. In murine models, it has been shown to be essential for deletion of autoreactive B cells in the germinal center. The role of Fas in human B-cell selection and in development of autoimmunity in patients carrying FAS mutations is unclear. We analyzed patients with either a somatic FAS mutation or a germline FAS mutation and somatic loss-of-heterozygosity, which allows comparing the fate of B cells with impaired vs normal Fas signaling within the same individual. Class-switched memory B cells showed: accumulation of FAS-mutated B cells; failure to enrich single V, D, J genes and single V-D, D-J gene combinations of the B-cell receptor variable region; increased frequency of variable regions with higher content of positively charged amino acids; and longer CDR3 and maintenance of polyreactive specificities. Importantly, Fas-deficient switched memory B cells showed increased rates of somatic hypermutation. Our data uncover a defect in B-cell selection in patients with FAS mutations, which has implications for the understanding of the pathogenesis of autoimmunity and lymphomagenesis of autoimmune lymphoproliferative syndrome.
Introduction
The autoimmune lymphoproliferative syndrome (ALPS) is associated with defects in components of the Fas (APO-1, CD95)-dependent signaling cascade, leading to apoptosis. Most patients with ALPS carry mutations in the gene encoding for Fas receptor, either heterozygous germline mutations (ALPS-FAS) or somatic mutations in cells of hematopoietic origin (ALPS-sFAS).1-3 In the case of FAS mutations with low penetrance, the onset of disease may be associated with the combination of germline and somatic events, because it has been observed in somatic loss of heterozygosity (ALPS-FAS-sLOH) and accumulation of second-site FAS mutations (ALPS-FAS-sFAS).4 The main clinical features of ALPS are lymphoproliferation associated with an accumulation of TCRαβ+CD4–CD8– T cells (double-negative [DNT] cells), antibody-mediated autoimmune cytopenia, and increased susceptibility to lymphoid malignancies.1,2,5,6 The study of ALPS patients therefore provides an excellent opportunity to investigate the molecular regulation of B-cell tolerance in humans.
In the germinal center (GC), antigen-activated B cells expressing high level of Fas7 receive costimulatory signals from follicular T-helper cells that provide CD40L and produce cytokines. Expression of the activation-induced deaminase (AID) induces somatic hypermutation (SHM), changing the affinity of antibodies to their cognate antigens.8 Because SHM may change the specificity of antibodies from nonautoreactive to autoreactive, the elimination of B cells in the course of the GC reaction by negative selection prevents the generation of autoreactive memory B cells and plasma cells and the development of autoimmunity. Several mechanisms regulate the selection in GCs. They include the quality and length of interactions with CD4+ T cells,8-10 changes in the B-cell receptor (BCR) affinity for the antigen,11,12 and Fas-dependent apoptosis.13 Whether Fas plays a B cell–intrinsic role in GC selection is a matter of debate because Fas-deficient MLRlpr/lpr mice and mice with conditional deletion of Fas in GC B cells develop a lupuslike disease with autoantibody production, lymphadenopathy, and splenomegaly.13,14 However, it has been shown that Fas is not required to eliminate conventional self-reactive B cells.15 Whether these observations in mice can explain autoimmunity in ALPS patients remains unclear.
It has been reported that ALPS patients have low numbers of circulating memory and marginal zone B-cell subsets,16 reduced polysaccharide antigen IgM responses,17 but increased BAFF serum levels.18 Although these findings suggest a role for Fas signaling in human B-cell physiology, it remains unclear whether these changes are related to the autoimmunity observed in ALPS patients, and how. To address this issue in more detail, we made use of 2 unique genetic constellations in which Fas-deficient and Fas-competent B cells can be studied in the same individual. First: ALPS-sFAS patients that carry both wild-type and Fas-deficient hematopoietic cells including B cells3,19-21 ; and second: ALPS-FAS-sLOH4 patients who have heterozygous germline mutations in the FAS gene affecting Fas expression, in which has occurred an additional loss-of-heterozygosity affecting the second allele in a proportion of their hematopoietic cells, leading to coexistence of B cells with reduced and absent Fas expression.
Comparing the selection and differentiation potential of Fas-competent and Fas-deficient B cells within one individual, we demonstrated that Fas plays a pivotal role in selecting GC B cells into the pool of memory B cells. This is the first evidence that Fas plays an important role in the GC reaction in humans with implications for the understanding of autoimmunity in ALPS patients.
Methods
Study approval
The study was approved by the institutional review board of the University of Freiburg (protocol number 40/09) and the University Hospital Carl-Gustav Carus Dresden (EK 320122008). Informed consent was obtained from the parents and/or the patients as well as the healthy donors. All experiments were carried out in accordance with the Declaration of Helsinki.
Patients’ description
Clinical data of patients P1 and P2 are described in supplemental material, available on the Blood Web site.
Flow cytometry and fluorescence-activated cell sorting
Phenotype of human B cells was determined with the following antibodies: CD27 FITC, CD69 FITC, CD3 FITC, CD24 PE, CD86 PE, CD95 PE, CD19 APC-H7, CD27 PE-Cy7, CD38 PE-Cy7, CD38 PerCP-Cy5.5 (Becton Dickinson); CD95 Alexa Fluor-647, HLA-DR APC, IgM APC-H7, IgD PE-Cy7, CD27 PerCP-Cy5.5 (Biolegend); IgA DyLight 649, IgG DyLight 649, IgM APC (Jackson Immunoresearch); and IgA PE, IgG PE (Southern Biotechnology). Dead cell exclusion was performed by 4,6 diamidino-2-phenylindole (DAPI) staining. B-cell proliferation was monitored by carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) labeling. The data were acquired with BD FACS Canto II and were analyzed with FlowJo software version 8.7 (TreeStar Inc.). Cell sorting was performed with Cell-sorter MoFlo Astrios (Beckman Coulter).
In vitro cell culture
Magnetic-activated cell sorted B cells (1.5 × 105 cells/mL) were stimulated with CD40L supernatant prepared as described22 in Iscove’s medium supplemented with 10% fetal calf serum, insulin, apo-transferrin, nonessential amino acids, glutamine, and reduced glutathione.
Apoptosis assay of Epstein-Barr virus lines
Apoptosis in Epstein-Barr virus (EBV) lines was induced by FasL and assessed by Annexin V/DAPI staining. Details are listed in the supplemental material.
Genetic analysis
In patients, FAS gene exons and exon-intron boundaries were sequenced using DNA from granulocytes, DNT cells, or clones of EBV-immortalized B cells as described.16 Sorted single B-cell DNA genotyping to detect heterozygous or homozygous FAS mutation in exon 1 was designed with the PyroMark Assay Design Software v.2.0.1.15 and performed on the PyroMark Q96 MD apparatus (QIAGEN). Further details of the reaction are listed in the supplemental material.
Generation of single-cell EBV-transformed B-cell clones
Magnetically separated human B cells (B-cell isolation kit II; Miltenyi) were fluorescence-activated cell-sorted (FACS) in naïve (CD3–19+27–IgG/A–) and switched memory (CD3–CD19+27+IgG/A+) subpopulations (supplemental Figure 1). Sorted cells were then incubated in complete medium containing 50% EBV supernatant (of B95-8 cells) and 2.5 μg/mL CpG ODN 2006 (Apara Biosciences) for 4 hours at 37°C. Cells were then seeded on 10 000 irradiated allogeneic mononuclear cells at 3 or 10 cells per well in 96 U-bottom plates.23 In this system, limiting dilution and the absence of FasL ensured that each cell could grow independently of FAS expression. Assessment of clonality is described in the supplemental material.
Enzyme-linked immunosorbent assay
Immunoglobulin content and specificity in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA). Details on the procedure are described in the supplemental material.
BCR repertoire analysis with next-generation sequencing
The VH-JH junctions were amplified in a multiplex polymerase chain reaction (PCR) by using the VH1-6 FR1 and JH consensus BIOMED-2 primers24 from sorted naïve and switched memory B cells. The primers were adapted for 454 sequencing by adding the forward A or reverse B adaptor, the “TCAG” key, and the multiplex identifier adaptor. PCR products were purified by gel extraction (QIAGEN) and Agencourt AMPure XP beads (Beckman Coulter). Subsequently, the PCR concentration was measured with the Quant-it Picogreen dsDNA assay (Invitrogen, Carlsbad, CA). The purified PCR products were sequenced on the 454 GS junior instrument according to the manufacturer’s recommendations by using the GS junior Titanium emPCR kit (Lib-A), sequencing kit, and PicoTiterPlate kit (Roche). By using the CLC genomic workbench software, the samples were separated based on their multiplex identifier sequence and trimmed, and reads with a quality score of <0.05 and <250 bp were discarded. The reads were uploaded to IMGT HighV-Quest software (International imMunoGeneTics information system, imgt.org).25 Subsequently, these output files were uploaded in the IGGalaxy tool.26 The percentage of SHM was calculated based on the data provided by IMGT. The number of mutations in the CDR1-FR3 region was divided by the number of nucleotides present in the CDR1-FR3. Mutations in the FR1 and CDR3 region were excluded from the analysis.
Statistical analysis
For the statistical analysis of gene frequencies, naïve and switched memory BCR variable region sequences were compared in 3 HDs, P1 and P2 (supplemental Tables 2 and 3). Details on statistical analysis of genes frequency are described in the supplemental material.
For the analysis of SHM rate, CDR3 length and polyreactivity data unpaired Student t test, Fisher’s exact test, and two-way analysis of variance were applied. The statistical analysis was performed with GraphPad Prism 6.
Results
ALPS patients with somatic mutations carry both Fas-competent and Fas-deficient B cells
Patient 1 (P1) carries a previously published heterozygous somatic splice-site FAS mutation (IVS7 c.652-1 G>A).21,27 The mutation results in the deletion of exon 8, causing a frame-shift and a premature stop codon in exon 9 compromising the Fas death domain. This predicts a dominant-negative effect on Fas-mediated apoptosis, whereas surface Fas expression is normal. Patient P228 carries a heterozygous germline mutation in the FAS gene, changing the start codon ATG into ATT in exon 1 (c.3G>T), leading to absent protein expression.28 This mutation results in haploinsufficiency and low Fas expression, but residual Fas signaling.
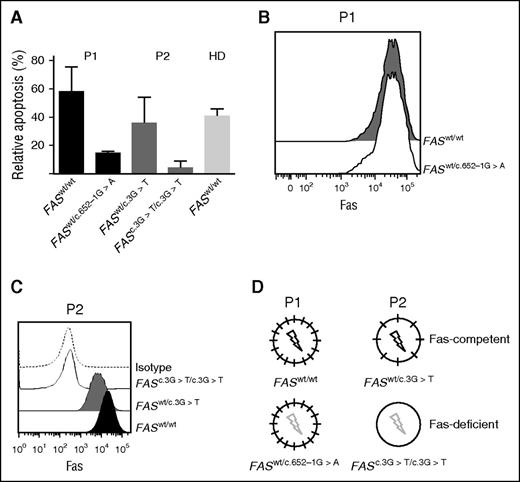
In both patients, the somatic mutation was originally detected in sorted DNT cells. To assess the presence of the somatic mutation also in the B lymphocytes, we performed single-cell EBV transformation23 of B cells isolated from P1 and P2, followed by FAS sequencing. Analysis of single-cell EBV clones from P1 revealed both wild-type (FASwt/wt) and heterozygous mutated (FASwt/c.652-1G>A) B cells. FASwt/c.652-1G>A EBV B cells showed defective Fas-dependent apoptosis (Figure 1A), but similar Fas expression as FASwt/wt B-cell clones (Figure 1B). Fas expression was reduced in P2 EBV B-cell clones carrying the heterozygous FASwt/c.3G>T mutation, and was absent in EBV B-cell clones that had lost the wild-type FAS allele caused by somatic LOH (FASc.3G>T/c.3G>T) (Figure 1C). As expected, FASwt/c.3G>T cells were still sensitive to Fas-induced apoptosis, whereas FASc.3G>T/c.3G>T cells were completely resistant (Figure 1A). In P2, the FASc.3G>T/c.3G>T B cells were easily identified by flow cytometry (Figure 1C). Based on these results, we defined the FASwt/wt B cells of P1 and the FASwt/c.3G>T B cells of P2 as Fas-competent, whereas FASwt/c.652-1G>A B cells in P1 and FASc.3G>T/c.3G>T B cells in P2 were defined as Fas-deficient (Figure 1D).
ALPS patients with somatic mutations carry both Fas-competent and Fas-deficient B cells. (A) Fas-induced apoptosis of EBV-transformed B-cell clones of P1, P2, and a healthy donor (HD). Both patients had B-cell populations sensitive as well as resistant to Fas-induced apoptosis (representative of 2 independent experiments). (B) Surface Fas expression in EBV-transformed B-cell clones of P1 and (C) P2. (D) Schematic depiction of Fas expression (short black line) and function (black flash, Fas-induced apoptosis normal; gray flash, Fas-induced apoptosis defective in P1 and P2).
ALPS patients with somatic mutations carry both Fas-competent and Fas-deficient B cells. (A) Fas-induced apoptosis of EBV-transformed B-cell clones of P1, P2, and a healthy donor (HD). Both patients had B-cell populations sensitive as well as resistant to Fas-induced apoptosis (representative of 2 independent experiments). (B) Surface Fas expression in EBV-transformed B-cell clones of P1 and (C) P2. (D) Schematic depiction of Fas expression (short black line) and function (black flash, Fas-induced apoptosis normal; gray flash, Fas-induced apoptosis defective in P1 and P2).
Accumulation of switched memory B cells carrying somatic FAS mutations
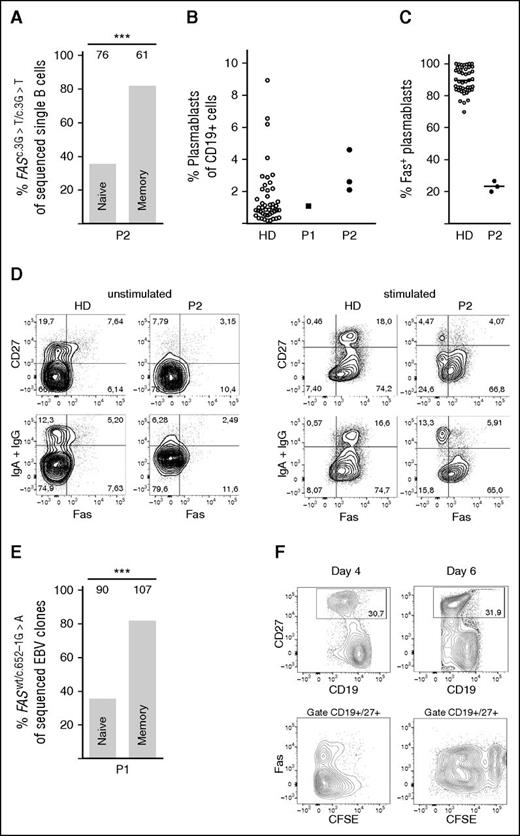
If Fas regulates the selection of activated B cells into the pool of memory B cells, the proportion of Fas-competent and Fas-deficient B cells should differ between naïve and switched memory B cells. Single-cell PCR analysis of ex vivo sorted cells from P2 (supplemental Figure 1B) revealed that 35.5% of naïve B cells carried the homozygous FAS mutation (FASc.3G>T/c.3G>T), although it was elevated to 82% in switched memory B cells (Figure 2A). Peripheral B cells express variable levels of Fas, lowest in naïve cells and homogeneously high in plasmablasts (supplemental Figure 2A-C). The preferential selection of Fas-deficient cells into the pool of post–GC-derived B cells was further supported by the strong enrichment of FASc.3G>T/c.3G>T cells in the pool of circulating plasmablasts (Figure 2B-C and supplemental Figure 2B). Because it is difficult to detect Fas expression in resting switched memory B cells, Fas was induced by overnight CD40L stimulation.29,30 In contrast to B cells from healthy controls, only one third of IgG+ or IgA+ switched memory B cells from P2 expressed Fas (Figure 2D). Similar data were obtained in P1. In this patient, we sorted naïve and memory B cells and performed single-cell EBV immortalization. The proportion of FASwt/wt and FASwt/c.652-1G>A cells was then determined by FAS sequencing of each individual clone. Notably, 90% of EBV clones generated from switched memory B cells of P1 carried mutations in FAS, whereas only 47% of the EBV clones derived from naïve cells were Fas-deficient (Figure 2E and supplemental Figure 1A). Therefore, Fas seems to play an important role in shaping the repertoire of post-GC memory B and plasma cells.
Accumulation of Fas-deficient cells among switched memory B cells. (A) Proportion of Fas-deficient B cells (FASc.3G>T/c.3G>T) among ex vivo FACS-sorted naïve or switched memory single B cells of P2, by single-cell PCR and sequencing of the specific FAS mutation. The numbers above the bars indicate number of sequenced single B cells. (B) Percent of plasmablasts in the CD19+ gate in blood of HD (n = 45), P1, and P2—3 independent measurements. (C) Percent of plasmablasts expressing Fas, measured in blood of a HD (n = 44) and P2 (3 independent measurements). Mean value indicated as a line. (D) Surface Fas expression on B cells of P2 and HD before and after 24-hour stimulation with CD40L, representative of 3 independent experiments. (E) Proportion of Fas-deficient (FASwt/c.652G>A), EBV-immortalized, single B-cell clones derived from FACS-sorted naïve or switched memory B cells of P1. The numbers above the bars indicate number of sequenced clones. (F) Fas-deficient and Fas-competent B cells show a similar proliferation profile. Proliferation by CFSE dilution of magnetically isolated B cells from P2 in response to stimulation with CD40L+IL-21 for 4 and 6 days. ***P < .001 (Fisher’s exact test).
Accumulation of Fas-deficient cells among switched memory B cells. (A) Proportion of Fas-deficient B cells (FASc.3G>T/c.3G>T) among ex vivo FACS-sorted naïve or switched memory single B cells of P2, by single-cell PCR and sequencing of the specific FAS mutation. The numbers above the bars indicate number of sequenced single B cells. (B) Percent of plasmablasts in the CD19+ gate in blood of HD (n = 45), P1, and P2—3 independent measurements. (C) Percent of plasmablasts expressing Fas, measured in blood of a HD (n = 44) and P2 (3 independent measurements). Mean value indicated as a line. (D) Surface Fas expression on B cells of P2 and HD before and after 24-hour stimulation with CD40L, representative of 3 independent experiments. (E) Proportion of Fas-deficient (FASwt/c.652G>A), EBV-immortalized, single B-cell clones derived from FACS-sorted naïve or switched memory B cells of P1. The numbers above the bars indicate number of sequenced clones. (F) Fas-deficient and Fas-competent B cells show a similar proliferation profile. Proliferation by CFSE dilution of magnetically isolated B cells from P2 in response to stimulation with CD40L+IL-21 for 4 and 6 days. ***P < .001 (Fisher’s exact test).
Fas-deficient B cells proliferate similarly to Fas-competent cells in vitro, but have longer proliferation history ex vivo
The accumulation of Fas-deficient memory B cells could be the result of increased proliferation after leaving the GC. We therefore compared the proliferation of CFSE-labeled B cells from P2 activated in vitro with CD40L and IL-21, because these ligands mimic best the activation of B cells in the GC reaction.31 In vitro, in absence of FasL, Fas-competent (FASwt/c.3G>T) and Fas-deficient (FASc.3G>T/c.3G>T) B cells proliferated similarly (Figure 2F).
Performing the KREC assay32 on P2 sorted B-cell subpopulations (supplemental Figure 1B), we found that Fas-deficient memory cells revealed a higher ratio between coding joint and signal joint (ΔCT = 3.12), compared with Fas-competent memory cells (ΔCT = 0.82) (supplemental Table 1). These data indicated that in vivo Fas-deficient memory cells underwent more rounds of proliferation then Fas-competent cells, possibly in the GC.
Disturbed repertoire among Fas-deficient memory B cells
The repertoire of B-cell receptors changes during B-cell differentiation because switched memory cells are significantly more oligoclonal than naïve B cells.33 To analyze the impact of Fas deficiency on the switched memory B-cell repertoire, we sorted naïve and switched memory B cells from P1 and P2 and from 3 healthy donors (HDs) (supplemental Figure 1 and supplemental Tables 2 and 3). In addition, in P2, the switched memory compartment was sorted by Fas expression into Fas-competent (FASwt/c.3G>T) and Fas-deficient (FASc.3G>T/c.3G>T) cells. The heavy (H-) chain variable region sequences of these populations were analyzed for the frequency of individual combinations of V-D and D-J, and for single V, D, or J gene segment frequency.
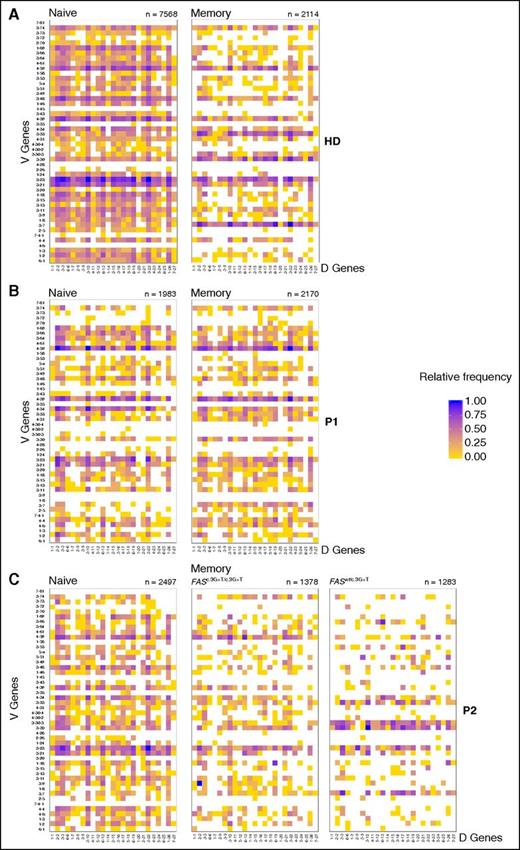
The repertoire of naïve B cells in HDs was composed of a large variety of unique combinations of differently rearranged V, D, J gene segments (Figure 3A and supplemental Figure 3A). Among switched memory cells, however, some combinations of V-D and D-J genes were found more frequently than others, whereas some combinations were not detected at all (Figure 3A and supplemental Figure 3A). These differences between naive and memory B cells were smaller in P1 and P2, especially for P2 Fas-deficient memory B cells (FASc.3G>T/c.3G>T) (Figure 3B-C and supplemental Figure 3B-C). Looking at the relative frequency of single V, D, J genes in the H-chain variable regions, we observed that certain V genes were represented with higher frequency compared with naïve cells in HDs-switched memory cells (supplemental Figure 4, arrows). In patients, we observed an increased frequency of distinct V genes only for Fas-competent memory cells of P2 (FASwt/c.3G>T) compared with naïve cells, but not for P1 (FASwt/c.652-1G>A) or for P2 (FASc.3G>T/c.3G>T) Fas-deficient cells (supplemental Figure 4).
Disturbed shaping of the repertoire in memory B-cell compartment. Heat map representing the frequency of V-D gene combinations in naïve and switched memory B cell of (A) healthy donors (HDs) and (B) P1 and (C) P2. For P2, memory B cells have been divided by Fas-deficient (FASc.3G>T/c.3G>T) and Fas-competent (FASwt/c.3G>T). Numbers below the map indicate the total number of productive sequences represented. Color scale indicates the relative frequency of each combination. Data obtained by deep sequencing of immunoglobulin variable region genes.
Disturbed shaping of the repertoire in memory B-cell compartment. Heat map representing the frequency of V-D gene combinations in naïve and switched memory B cell of (A) healthy donors (HDs) and (B) P1 and (C) P2. For P2, memory B cells have been divided by Fas-deficient (FASc.3G>T/c.3G>T) and Fas-competent (FASwt/c.3G>T). Numbers below the map indicate the total number of productive sequences represented. Color scale indicates the relative frequency of each combination. Data obtained by deep sequencing of immunoglobulin variable region genes.
The differences in gene frequency were quantified by calculating the standard deviations (SD) of the true differences in the distribution of each gene between naïve and memory cells (supplemental Tables 4 and 5). A larger SD indicates a larger difference in the representation of single V, D, J genes between naïve and memory H-chain variable regions and suggests preferential selection of certain V, D, J genes in the memory compartment. In HDs, the SD was highest for V genes (range, 1.234-3.300), and of similar magnitude for D (range, 0.538-2.260) and J genes (SD range, 0.559-2.341). In P1 and P2, much lower standard deviations were observed for all 3 genes when comparing naïve with all switched memory cells. This indicated a more similar distribution of the genes between the pre- and post-GC cells and therefore less efficient selection in the switched memory compartment compared with controls. However, in P2 when comparing separately Fas-deficient (FASc.3G>T/c.3G>T) and Fas-competent (FASwt/c.3G>T) memory cells with naïve cells, much higher SD were observed. In particular, the differences in frequency of single genes in the comparison between Fas-competent (FASwt/c.3G>T) cells and naïve cells was of a magnitude similar to the one observed in HDs, indicating a more favorable outcome in the shaping of the repertoire in Fas-competent cells.
Fas-induced apoptosis prevents the development of autoreactive memory B cells
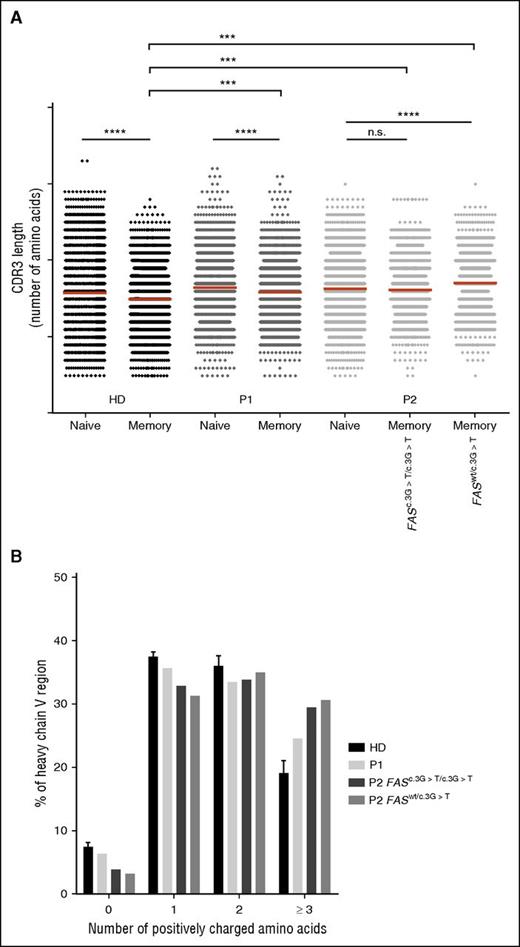
In HDs, the CDR3-encoding regions of functionally rearranged VDJ gene segments expressed by naïve B cells are longer than those of switched memory B cells.34 Shortening of CDR3 was also observed between naïve and memory cells in P1, whereas in P2, CDR3 of memory cells were as long as those of naïve B cells (Figure 4A). Nevertheless, memory cells of both patients had significantly longer CDR3 compared with healthy donors.
Longer CDR3 sequences and increased representation of positively charged amino acids in switched memory B cells. (A) Amino acid length of CDR3 sequences in FACS-sorted naïve and switched memory B cells from HD (data pooled from 3 individuals), P1, and P2 analyzed by deep sequencing; ***P < .001 and ****P < .0001 (unpaired Student t test). (B) Distribution of number of positively charged amino acids (0, 1, 2, ≥3) per H-chain V region of sorted switched memory B-cell in HD (data pooled from 3 individuals), P1, and P2 analyzed by deep sequencing. In P2, FAS-competent (FASwt/c.3G>T) and FAS-deficient (FASc.3G>T/c.3G>T) memory cells are analyzed separately.
Longer CDR3 sequences and increased representation of positively charged amino acids in switched memory B cells. (A) Amino acid length of CDR3 sequences in FACS-sorted naïve and switched memory B cells from HD (data pooled from 3 individuals), P1, and P2 analyzed by deep sequencing; ***P < .001 and ****P < .0001 (unpaired Student t test). (B) Distribution of number of positively charged amino acids (0, 1, 2, ≥3) per H-chain V region of sorted switched memory B-cell in HD (data pooled from 3 individuals), P1, and P2 analyzed by deep sequencing. In P2, FAS-competent (FASwt/c.3G>T) and FAS-deficient (FASc.3G>T/c.3G>T) memory cells are analyzed separately.
Self-reactivity of antibodies is associated with the presence of positively charged amino acids in the antigen-binding portion of the variable region of the immunoglobulin H-chain because positive charges facilitate binding to negatively charged self-antigens like DNA or carbohydrates.35-38 Although antinuclear antibodies were absent in both patients, we found an increase in the number (≥3) of positively charged amino acids in the H-chain variable regions in memory B cells of P1 and P2, both in Fas-competent and Fas-deficient cells (Figure 4B). Indeed, both patients had antibodies against red blood cells antigens, which are often glycosylated proteins or carbohydrates themselves.39 Interestingly, CDR3 with positively charged aminoacids were enriched in the naïve B-cell compartment of both patients compared with controls (supplemental Figure 5).
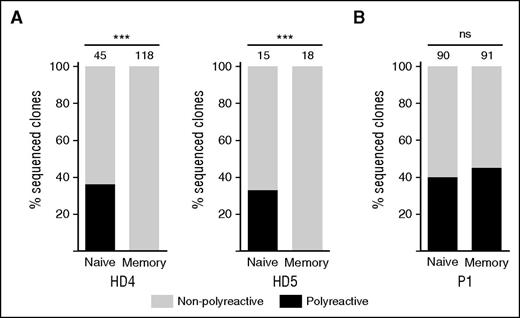
Normally, switched memory B cells are less polyreactive than naïve B cells because somatic hypermutation and selection favors cells expressing antibodies with high affinity for a specific antigen.40,41 We found that similar percentages of EBV-immortalized clones generated from naïve B cells from 2 HDs and P1 expressed polyreactive antibodies (Figure 5A-B and supplemental Figure 6). In contrast, polyreactive antibodies were not secreted by clones derived from switched memory B cells of HDs (Figure 5A), although the percentage of polyreactive clones derived from memory B cells of P1 was similar to naïve cells (Figure 5B). Of the 41 polyreactive clones derived from switched memory cells, 35 (85%) were Fas-deficient (FASwt/c.652-1G>A).
Defective clearance of polyreactive B cells in germinal centers. The proportion of polyreactive (black bar) and nonpolyreactive (white bar) single-cell EBV immortalized B-cell clones from sorted B-cell subpopulations in (A) 2 HD and (B) P1. The numbers above the bars indicate numbers of clones analyzed. Polyreactivity was defined as positive ELISA titer in the same clone to ≥3 antigens. ***P < .001 (Fisher’s exact test).
Defective clearance of polyreactive B cells in germinal centers. The proportion of polyreactive (black bar) and nonpolyreactive (white bar) single-cell EBV immortalized B-cell clones from sorted B-cell subpopulations in (A) 2 HD and (B) P1. The numbers above the bars indicate numbers of clones analyzed. Polyreactivity was defined as positive ELISA titer in the same clone to ≥3 antigens. ***P < .001 (Fisher’s exact test).
SHM in the V regions promote the selection of highly antigen-specific GC B cells into the pool of long-lived switched memory B cells.41 The number of SHM per V-region is dependent on the rounds of proliferation in the GC.42 We found a significant increase in SHM in P1 and P2 Fas-deficient memory B cells compared with HD memory cells. In P2, Fas-competent memory B cells had similar SHM as HD controls (Figure 6). These results together with the observation of longer proliferation history of Fas-deficient memory cells may suggest that Fas-induced apoptosis limits the cycling of GC B cells, thereby preventing the evolution of autoreactive specificities among switched memory B cells.
Increased SHM per variable H-chain in Fas-deficient B cells. Somatic hypermutations per V region were calculated as the number of mutations in the CDR1-FR3 region per number of nucleotides present in the CDR1-FR3 in switched memory B cells of HD (data pooled from 3 donors), P1, and P2. ****P < .001 (2-way analysis of variance).
Increased SHM per variable H-chain in Fas-deficient B cells. Somatic hypermutations per V region were calculated as the number of mutations in the CDR1-FR3 region per number of nucleotides present in the CDR1-FR3 in switched memory B cells of HD (data pooled from 3 donors), P1, and P2. ****P < .001 (2-way analysis of variance).
Discussion
Although antibody-mediated autoimmunity including autoimmune cytopenia is a major manifestation of ALPS, the contribution of Fas deficiency to the development and selection of autoreactive B cells in humans has not yet been investigated. We studied the repertoire and antigen specificity of switched memory B cells in 2 ALPS patients with somatic FAS mutations. In both patients, somatic mutations in the FAS alleles allowed Fas-deficient and Fas-competent B cells to develop side-by-side in a single individual. This particular setting minimizes the influence of the individual infection history, environmental changes, and differences in the genetic background on the composition of the repertoire of antigen-selected switched memory B cells.
In both patients, the majority of switched memory B cells were Fas-deficient, although the naïve B-cell compartment, which forms the reservoir of the precursors of the switched memory B cells, contained similar proportions of Fas-deficient and Fas-competent B cells. We found a similar pattern of proliferation in vitro in Fas-competent and Fas-deficient B cells, but longer proliferation history by KRECS in Fas-deficient B cells ex vivo, indicating that, similar to DNT cells,43,44 impaired apoptosis and proliferative advantage appear relevant for the accumulation of Fas-deficient memory B cells.
Recent findings in mouse models implicate an intrinsic role for Fas signaling in the negative selection of so-called GC rogue B cells. These are characterized by high SHM that leads to unchecked production of autoantibodies secreting plasma cells.15 Fas-deficient memory cells of both ALPS patients show similar feature to rogue B cells because they have a higher chance to develop into switched memory B cells than Fas-competent B cells, carry highly mutated B-cell receptors, and accumulate also in the plasma cell compartment. Compared with HDs, patients’ memory B cells expressed immunoglobulin V regions with longer CDR3s. Furthermore, their V region contained more positively charged amino acid residues. Because positively charged CDRs are associated with autoreactive antibodies,35-38 patients’ B cells seem to have escaped the negative selection of self-reactive B cells in the GC reaction. In line with the model proposed by Goodnow,45 Fas deficiency in B cells would represent a first event in the breakdown of tolerance, and additional events are necessary for the manifestation of autoimmune disease. In fact, both Fas-competent and Fas-deficient switched memory cells of P2 showed longer CDR3 and an increased number of positively charged amino acids, suggesting that the GC of ALPS patients has a more complex defect in selection. In this context, Fas-deficient T cells may play a role in addition to the intrinsic defect in B cells.14 Nevertheless, as for rogue GC B cells,15 the resistance to apoptosis in Fas-deficient B cells is likely to be a key factor for their accumulation in the switched memory compartment.
The role of Fas as a gatekeeper preventing autoreactive GC cells from entering the pool of memory B cells was further substantiated by the high frequency of polyreactive IgG antibodies secreted from single clones in Fas-deficient EBV lines isolated from the switched memory B-cell pool of P1. In HDs, B cells expressing polyreactive IgM and IgD antibodies represent a significant fraction of transitional and naïve B cells,46 but they are lost when GC B cells expressing IgG or IgA antibodies with high affinities for non–self-antigens are selected into the switched memory B-cell compartment.47,48 In P1, the frequency of EBV clones secreting polyreactive IgM antibodies was as high as the frequency of clones secreting IgG.
Although Fas-deficient, naïve B cells expressing many different kinds of self-reactive antibodies seem to be easily selected into the switched memory B-cell pool, both ALPS patients reported here have lower numbers of circulating switched memory B cells than matched control individuals. Similar observations have also been made before, even though the total number of circulating B cells was normal or even increased.16,49 An explanation for this seemingly paradox observation might be provided by the high level of SHM in Fas-deficient switched memory B cells compared with Fas-competent memory cells. In fact, Fas-deficient B cells of MRLlpr/lpr mice express elevated levels of AID catalyzing SHM and the formation of DNA double-strand breaks that are required to initiate immunoglobulin class-switch recombination. Therefore, MRLlpr/lpr mice show a higher frequency of class switch recombination50 and of SHM51 than do wild-type mice. Our finding of increased SHM in memory B cells of P1 and in P2 is compatible with the idea that Fas signaling and Fas-induced apoptosis removes B cells that have accumulated somatic mutations. Indeed SHM may result not only in self-reactive specificities, but also in dysfunctional immunoglobulins or in double-strand breaks inside or outside the H-chain loci that cannot be repaired by the normal DNA repair machinery. Indirect evidence for this scenario comes from the observation that ALPS patients do not only develop autoimmunity but also B cell–derived lymphomas.19,20,49,52 Its cumulative incidence increases with age in the patients as well as in the healthy carriers of FAS mutations.19 As the SHM machinery also hits off-target genes,53,54 the increased SHM rate in Fas-deficient B cells may cause many chromosomal alterations, resulting in premature cell death of GC B cells. This would reduce the output of switched memory B cells and explain the high incidence of B-cell lymphomas in Fas-deficient ALPS patients.
Memory B cells expressing autoantibodies can be targeted by anti–B cell antibodies. Attempt to treat refractory cytopenia with rituximab in 14 ALPS patients resulted in a temporary positive effect in 9 patients and failed to induce remission in the remaining 5.55-57 Our analysis shows that most of the plasma cells of P2 were Fas-deficient. This suggests that Fas signaling plays a role also in the selection of GC B cells into the pool of long-lived plasma cells, similar to switched memory B cells. Because rituximab treatment does not affect plasma cells, the insufficient therapeutic outcome may be caused by the survival of autoantibody producing Fas-deficient plasma cells.
In conclusion, our results provide strong evidence for the Fas-dependent removal of autoreactive and polyspecific GC B cells in humans. In addition, Fas-dependent signals seem to limit the rounds of replication and the time window for somatic mutations and double-strand DNA breaks introduced by AID activity. Therefore, Fas may act as a gatekeeper, limiting the survival of GC B cells with genomic aberrations and ensuring the quality of the switched memory B cell and plasma cell compartment. Fas deficiency contributes significantly to the formation of autoreactive switched B cells in ALPS, suggesting that therapeutic approaches that specifically target plasma cells may have beneficial effects in Fas-deficient ALPS patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank B. Fischer, K. Fischer, S. Koch, and M. Schlaier for technical assistance; and J. Bodinek-Wersing from the CCI FACS Sorting Facility.
This study was supported by the German Federal Ministry of Education and Research grants BMBF 01EO1303, BMBF 01EO0803, and BMBF 01GM1111B (C.S., S.E., A.R.-E.); the “Margarete von Wrangell Fellowship” by the Ministry of Sciences, Research, and Arts in Baden Württemberg (M.R.); the DFG through SFB1160 (TP4); an unrestricted Baxter-ESID fellowship (A.J.); and the CAPES Foundation, Ministry of Education of Brazil, Brasilia/DF 70040-020 (2610/13-2) (R.L.).
Authorship
Contribution: A.J., K.S., M.v.d.B., H.E., and M.R. planned experiments, analyzed data, and wrote the manuscript; A.J. and M.R. conceived the study and finalized the manuscript; W.V. performed statistical analysis and wrote the manuscript; A.J., H.I., M.R.L., M.E., K.P., P.F., J.H., R.L., and M.S. planned, performed, and analyzed experiments; and J.R., F.H., C.S., A.R.-E., S.E., and E.T. provided patient material and critically read the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marta Rizzi, Center for Chronic Immunodeficiency and Clinic for Rheumatology and Clinical Immunology, University Medical Center Freiburg, Hugstetter Strasse 55, 79106 Freiburg, Germany; e-mail: marta.rizzi@uniklinik-freiburg.de.
References
Author notes
K.S. and M.v.d.B. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal