Abstract
Neutrophils are polymorphonuclear leukocytes of the phagocytic system that act as first line of host defense against invading pathogens but are also important mediators of inflammation-induced injury. In contrast to other members of the innate immune system, neutrophils are classically considered a homogenous population of terminally differentiated cells with a well-defined and highly conserved function. Indeed, their short lifespan, the absent proliferative capacity, their limited ability to produce large amounts of cytokines, and the failure to recirculate from the tissue to the bloodstream have sustained this idea. However, increasing evidence over the last decade has demonstrated an unexpected phenotypic heterogeneity and functional versatility of the neutrophil population. Far beyond their antimicrobial functions, neutrophils are emerging as decision-shapers during innate and adaptive immune responses. These emerging discoveries open a new door to understand the role of neutrophils during homeostatic but also pathogenic immune processes. Thus, this review details novel insights of neutrophil phenotypic and functional heterogeneity during homeostasis and disease.
One neutrophil or many?
Myeloid and lymphoid members of the hematopoietic system are composed of subsets with distinct phenotypic and functional characteristics. As an example, a wide variety of monocyte1 and macrophage2 subpopulations has been identified that display a unique transcriptional profile defining their function during homeostasis and immune responses.1 The diversity of tissue macrophages can partly be explained by their differential embryonic origin.3 Beyond ontogeny, macrophage heterogeneity is dictated by environmental signals inducing differentiation or activation.4 The recognition of external cues by macrophages triggers different patterns of epigenetic modifications, gene expression, and protein synthesis and instructs them to exert a specific function in homeostasis4 or inflammation.5 Such activation has been modeled as 2 opposing phenotypic states with different functional properties: M1 macrophage polarization stimulates Th1 effector and microbicidal responses, and M2 macrophage polarization fosters a Th2 and reparative response. Multiple studies have identified these phenotypes in different pathological situations and demonstrated their importance in disease outcome.2 The dichotomous view of macrophage activation, however, is shifting toward a more plastic understanding of macrophage polarization where macrophages can exhibit multiple phenotypic and functional signatures combining the responses to different environmental stimuli that may vary in a spatiotemporal fashion. This new concept has recently been exemplified in tissue-resident4 or tumor-associated macrophages.6 Thus, heterogeneity and plasticity of leukocyte subsets integrate ontogenic and environmental imprints.
In contrast to other leukocyte populations, the idea of neutrophil heterogeneity has received less attention, likely based on the traditional view of unique properties of neutrophils: Limited lifespan, reduced transcriptional activity, and inability to return to the circulation after migrating to extravascular tissue. Hence, we here review emerging evidence challenging these classical concepts of neutrophil biology and further discuss how this may set the foundation for neutrophil heterogeneity.
An adaptable lifespan
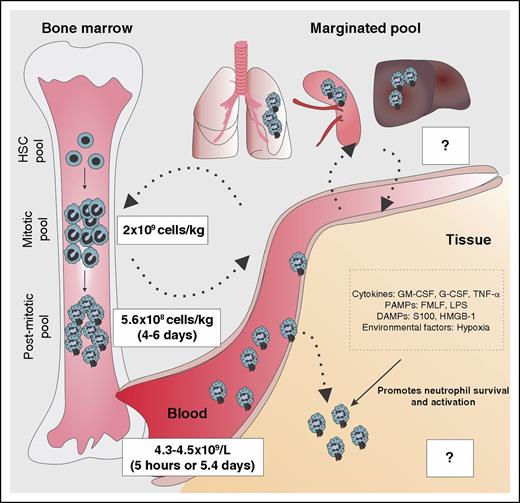
Although the lifespan of circulating neutrophils is classically thought to be short,7 this view was recently challenged by a study estimating the lifespan of human neutrophils to be 5.4 days.8 Hence, this longer lifespan of neutrophils may set the basis for neutrophils to undergo phenotypic and functional changes. To further understand neutrophil kinetics, assessment of neutrophil lifespan within tissues is of importance (Figure 1). At sites of inflammation, neutrophil lifespan is increased through inhibition of cell apoptosis, an effect triggered by cytokines, pathogen-associated molecular patterns (PAMPs), damage-associated molecular pattern molecules (DAMPs), or environmental factors.9,10 Indeed, in chronic inflammation, neutrophil lifespan is abnormally prolonged, thereby worsening disease prognosis. Extended neutrophil lifespan through decreased apoptosis is observed in patients with asthma11 with acute coronary syndrome12 and results in increased disease severity. Lifespan extension through prosurvival signals produced under inflammatory conditions may also increase the capacity of neutrophils to undergo phenotypic and functional changes and account for neutrophil heterogeneity. For example, oxygen deprivation in tissue during inflammation drives hypoxia-inducible factor–dependent activation of neutrophil prosurvival pathways13,14 and directly impacts on the bactericidal activity of neutrophils.13 Similarly, in the low oxygen and nutrient environment of cystic fibrosis airways, neutrophils undergo metabolic reprogramming,15 trigger prosurvival mechanisms,16 and acquire distinct phenotypic and functional properties (supplemental Discussion 1, available on the Blood Web site).
Integration of factors determining neutrophil lifespan. The bone marrow neutrophil lineage is composed of 3 compartments: (1) the hematopoietic stem cell (HSC) pool, (2) the mitotic pool that is comprised of myeloblasts, promyelocytes, and myelocytes, and (3) the postmitotic pool that is comprised of metamyelocytes, band cells, and mature neutrophils. The size of the mitotic and postmitotic pool is estimated at 4.36 × 109 and 8.8 × 109 cells/kg, respectively. After 4 to 6 days in the bone marrow, neutrophils are released to the bloodstream (4.3-4.5 × 109 cells/L). The estimated period of time where neutrophils circulate is controversial. Although some studies suggest that the circulating lifespan is around 5 hours, alternative experimental calculation raises neutrophil lifespan up to 5.4 days, as reported in different studies. Neutrophils are also located in the “marginated pools,” vascular pools located in the lungs, spleen, and liver where the turnover time is still unclear. After neutrophils infiltrate tissues during inflammation, interaction with tissue-derived signals such as cytokines, PAMPs, DAMPs, or environmental factors reduces neutrophil apoptosis and increases their lifespan to an extent that remains unknown. fMLF, formyl-methionyl-leucyl phenylalanine; HMGB1, high-mobility group box-1.
Integration of factors determining neutrophil lifespan. The bone marrow neutrophil lineage is composed of 3 compartments: (1) the hematopoietic stem cell (HSC) pool, (2) the mitotic pool that is comprised of myeloblasts, promyelocytes, and myelocytes, and (3) the postmitotic pool that is comprised of metamyelocytes, band cells, and mature neutrophils. The size of the mitotic and postmitotic pool is estimated at 4.36 × 109 and 8.8 × 109 cells/kg, respectively. After 4 to 6 days in the bone marrow, neutrophils are released to the bloodstream (4.3-4.5 × 109 cells/L). The estimated period of time where neutrophils circulate is controversial. Although some studies suggest that the circulating lifespan is around 5 hours, alternative experimental calculation raises neutrophil lifespan up to 5.4 days, as reported in different studies. Neutrophils are also located in the “marginated pools,” vascular pools located in the lungs, spleen, and liver where the turnover time is still unclear. After neutrophils infiltrate tissues during inflammation, interaction with tissue-derived signals such as cytokines, PAMPs, DAMPs, or environmental factors reduces neutrophil apoptosis and increases their lifespan to an extent that remains unknown. fMLF, formyl-methionyl-leucyl phenylalanine; HMGB1, high-mobility group box-1.
Transcriptional plasticity: numbers matter
Leukocyte differentiation toward functionally distinct subpopulations requires the ability of these cells to synthesize and release a new set of proteins in response to environmental signals. Although mature neutrophils are classically considered to have limited capacity for de novo protein synthesis,17 recent work has explored the magnitude and mechanisms of neutrophil transcriptional18 and translational regulation.19 It is important to recognize that human neutrophil mRNA content is 10 to 20 times lower than that of other leukocytes,20 which translates into a lower protein production on a per cell basis. However, because neutrophils recruited to inflamed tissues greatly outnumber other leukocytes, the overall impact of neutrophil-derived cytokines in the inflammatory response can be significant. Beyond cytokines, de novo protein synthesis in neutrophils extends to membrane receptors (eg, Fcγ receptor-121 ), which may additionally alter neutrophil heterogeneity and function. In line with the ability of neutrophils to synthesize de novo proteins, recent studies report an active chromatin remodeling and a fine regulation of mRNA expression. On one side, neutrophil gene expression is controlled on an epigenetic level by chromatin histone modification such as methylation22 or acetylation,23 processes that tightly influence gene transcription. On the other side, neutrophils display a cell-specific pattern of noncoding regulatory regions,24 elements that are involved in the regulation of gene expression. Interestingly, interleukin (IL)-6 expression by human neutrophils after exposure to Toll-like receptor (TLR)-8 ligands involved chromatin remodeling and increased activity of noncoding regulatory elements (eg, enhancers).23 Thus, this evidence supports an inducible epigenetic and genetic regulation of neutrophil gene expression. Moreover, the association between genetic variants24 and altered methylation profiles25 in neutrophils associated with disease susceptibility emphasizes the importance of understanding how gene expression is controlled in neutrophils.
Collectively, these studies indicate that neutrophils are endowed with an active transcriptomic function, the disturbance of which may provoke abnormal gene expression and impact on disease outcome. Moreover, neutrophil transcriptional modulation during communication with the immune network or interaction with environmental signals may dictate the function required in a specific situation. For instance, IL-13+ neutrophils prime macrophages toward an anti-inflammatory phenotype that enhances parasite clearance during nematode infection (supplemental Discussion 2). Similarly, neutrophil-derived IL-12 or IL-10 secreted by CD49d+ neutrophil subsets shape macrophage function during bacterial infection (supplemental Discussion 3).
Multidirectional migration
Abluminal to luminal transmigration
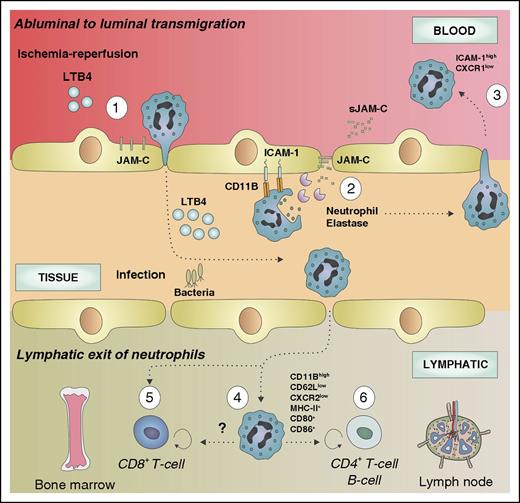
During the last decade, a number of studies have reported the ability of neutrophils to return to the bloodstream after migrating to the extravascular space,26-28 thus challenging the classical concept of unidirectional neutrophil migration. In vitro, reversely migrated neutrophils were shown to display a different phenotype (intercellular adhesion molecule-1 [ICAM-1]highCXCR1low) compared with circulating (ICAM-1lowCXCR1high) or tissue-resident (ICAM-1lowCXCR1low) neutrophils, a phenotype also found in low abundance in the circulation.26 Interestingly, reverse-transmigrated neutrophils were not able to transmigrate again into the tissue and had a prolonged lifespan and an increased capacity to produce superoxide species.26 The in vivo proof of reverse transmigration was described using intravital microscopy.28 Although luminal to abluminal transmigration is regulated by various junctional proteins (eg, platelet endothelial cell adhesion molecule-1, VE-cadherin, and CD99), reverse transmigration predominantly depends on junctional adhesion molecule (JAM)-C.28 Under ischemia-reperfusion, JAM-C is redistributed to a nonjunctional membrane location triggering neutrophil adhesion during luminal to abluminal migration,29 an event that was also observed after leukotriene B4 (LTB4) treatment. On the contrary, excessive LTB4 induced presentation of neutrophil elastase on CD11b, JAM-C degradation, and subsequent reverse transmigration (Figure 2).27 Neutrophils undergoing reverse transmigration exhibited a proinflammatory phenotype characterized by a high ICAM-1 expression.28 Interestingly, ICAM-1high neutrophils were found in distant organs after ischemia/reperfusion or LTB4 infusion, suggesting a potential implication in the systemic propagation of inflammation.27,28 Overall, reverse transmigration prolongs neutrophil lifespan and modulates their phenotype and function, thereby contributing to neutrophil heterogeneity.
Mutidirectional neutrophil migration. Neutrophil reverse transmigration from the subendothelial space to the circulation occurs predominantly after ischemia/reperfusion injury. After LTB4-driven neutrophil recruitment (1), infiltrated neutrophils interact with endothelial cells via CD11b and release NE, which degrades the JAM-C (2), allowing their circulation back to the bloodstream (3). Reverse-transmigrated neutrophils exhibit a distinct phenotype characterize by ICAM-1highCXCR1low. During pathogen-driven inflammation, infiltrated neutrophils may also return to lymphoid organs (bone marrow or lymph node) through the circulation or the lymphatic system. Inside the lymph nodes, neutrophils display an activated phenotype (CD11Bhigh, CD62Llow, CXCR2low) and express MHC (CH)-II and the costimulatory molecules CD80 and CD86, suggesting a newly acquired ability to present antigens (4). Furthermore, this subpopulation of neutrophils is able to modulate the adaptive immune response by promoting or repressing the T- and B-cell function in the lymph node (6). Neutrophils migrating toward the bone marrow induce CD8+ T cell–dependent antiviral responses (5).
Mutidirectional neutrophil migration. Neutrophil reverse transmigration from the subendothelial space to the circulation occurs predominantly after ischemia/reperfusion injury. After LTB4-driven neutrophil recruitment (1), infiltrated neutrophils interact with endothelial cells via CD11b and release NE, which degrades the JAM-C (2), allowing their circulation back to the bloodstream (3). Reverse-transmigrated neutrophils exhibit a distinct phenotype characterize by ICAM-1highCXCR1low. During pathogen-driven inflammation, infiltrated neutrophils may also return to lymphoid organs (bone marrow or lymph node) through the circulation or the lymphatic system. Inside the lymph nodes, neutrophils display an activated phenotype (CD11Bhigh, CD62Llow, CXCR2low) and express MHC (CH)-II and the costimulatory molecules CD80 and CD86, suggesting a newly acquired ability to present antigens (4). Furthermore, this subpopulation of neutrophils is able to modulate the adaptive immune response by promoting or repressing the T- and B-cell function in the lymph node (6). Neutrophils migrating toward the bone marrow induce CD8+ T cell–dependent antiviral responses (5).
Lymphatic transit of neutrophils
An alternative way of neutrophil emigration from the tissue involves the exit through the lymphatic vessels, a route that gives neutrophils access to secondary lymphoid organs (Figure 2). During infection, mouse neutrophils are able to migrate to the lymph node through lymph and blood vessels.30-36 The fact that neutrophils were detected carrying living bacteria from infected tissue to the lymph node underlines their migratory capacity and their potential to interact with the adaptive immune system.30 In agreement with these observations, Hampton and colleagues recently confirmed the ability of neutrophils to migrate into the lymph nodes after skin infection.34 Interestingly, migrated neutrophils exhibited a distinct activated phenotype (CD11Bhigh, CD62Llow, CXCR2low), which was required for migration to the lymph nodes. Functionally, specific blocking of neutrophil migration to the lymph nodes reduced T-cell proliferation, thereby demonstrating the importance of neutrophils in regulating the adaptive response.34 Accordingly, neutrophils were able to enter lymph nodes in a mouse model of antigen immunization and to modulate CD4+ T-cell and B-cell responses.36 Another example of neutrophil recircularization comes from the observation that neutrophils egress from virus-infected skin and infiltrate the bone marrow to interact with resident CD8+ T cells resulting in enhanced antiviral responses.32 Hence, recirculation of neutrophils to draining lymph nodes may act as an early step of antigen presentation and initiation of adaptive immune responses. In accordance with this, neutrophils recruited into lymph nodes upregulated major histocompatibility complex (MHC)-II and costimulatory molecules (CD80, CD86), which may be involved in neutrophil-dependent antigen presentation.34 Alternatively, it has been proposed that neutrophils carrying bacteria or viruses may serve as “Trojan horses” that permit the dissemination of the infection on macrophage engulfment.37,38 Collectively, the ability of neutrophils to egress from tissue, to re-enter into the circulation, and to migrate into secondary lymphoid organs represents 1 source of neutrophil plasticity and heterogeneity.
Neutrophil heterogeneity
Recent reviews have listed various forms of neutrophil heterogeneity.39,40 Below we discuss the most recent studies describing neutrophil heterogeneity under homeostatic and disease conditions (Table 1).41-72
Neutrophil heterogeneity in homeostatic and pathological conditions
| Neutrophil subset . | Prevalence . | Phenotypic properties . | Functional properties . | Homeostatic/pathological relevance . | Reference . |
|---|---|---|---|---|---|
| CD177+ | Human: ∼50% of circulating neutrophils | CD177+ Proteinase 3 | = Apoptosis = transmigration in human peritonitis↓ granule protein mRNA content | CD177 autoantibodies in ANCA-derived vasculitis patients | 41,,,-45 |
| “Aged” | Mouse: | CXCR4highCD62LlowCD11Bhigh CD49high; hypersegmented nucleus; Reduced size and granularity | Regulation of stem cell niche and circadian release of HSPCs to the circulation / Enhanced intravascular inflammation | 46,47 | |
| 55 ± 14% in ZT5 (clearance from the circulation period) | = Apoptosis | ||||
| 8 ± 3% in Z17 (release from the circulation period) | ↑ Phagocytosis and NETosis | ||||
| OLFM4+ | Human: 20-25% of circulating neutrophils | OLFM4 expression in neutrophil-specific granules | = Apoptosis | OLFM4 autoantibodies in ANCA-derived vasculitis patients | 48,,,-52 |
| = Phagocytosis | Enhanced immune response against S aureus and E coli infection in absence of OLFM4 | ||||
| = Transmigration | |||||
| ↓ Cathepsin C activity | |||||
| OLFM4 + NET formation | |||||
| TCR+ | Human: 3-5% of circulating neutrophils | Human: TCRα,β variants | Delayed apoptosis and IL-8 production on CD3/CD28 stimulation | Reduced TCR variants in aged subjects compared to young individuals | 53,54 |
| Mouse: Vα2, Vα5, Vβ1, Vβ16 | |||||
| Angiogenic | Mouse: 2.8% | CD49d+VEGFR1highCXCR4high | ↑MMP9 | Promotes angiogenesis in hypoxic tissue | 55,56 |
| Human: 3.2% | |||||
| CD62Ldim/CD16brightCD62Lbright/CD16dim | Human: 3 hours after LPS administration: | CD62Lbright; CD16bright; multi-lobular nucleus | CD62Ldim/CD16bright: -Inhibition of T-cell proliferation through ROS release and dependent on CD11B expression | Potential implication in sepsis-related immunosuppresion | 57,58 |
| CD62Lbright/CD16bright: 60-70% | CD62Ldim; CD16bright; CD11Chigh; CD11Bhigh; CD54high ; hypersegmented nucleus | ||||
| CD62Ldim/CD16bright:20-25% | CD62Lbright; CD16dim; CD11Clow; CD11Blow; CD54low ; band-form nucleus | ||||
| CD62Lbright/CD16dim:10-15% | |||||
| CD63+ | Human: neutrophils isolated from lung sputum | CD11Bhigh CD66high CD63+CD80+MHC-II+ | ↓ Glutathione activity | Neutrophil elastase-dependent perpetuation of inflammation, infection and progression of cystic fibrosis airway disease | 59,60 |
| ↑ Neutrophil elastase activity | |||||
| ↑ Arg1 | |||||
| IL-13+ | Not defined | Ring-form nucleus; IL-5; IL-13; IL-33; insulin-like growth factor-1; Resistin-like molecule alpha/FIZZ1; chitinase-like 3. | Priming of macrophages towards alternative or M2 phenotype | Accelerated clearance of nematodes by primed macrophages | 61 |
| CD49+ | Mouse: | PMN-I: CD11B−, CD49+, TLR2+, TLR4+, TLR5+, TLR8+; multilobular nucleus, MPOhigh | PMN-I: IL-12, CCL3, classic activation of macrophages | CD49d+ neutrophils promote virus-challenged experimental asthma | 62,-64 |
| Sendai virus infection: 50% of BAL neutrophils | |||||
| MRSA infection: not defined | PMN-II: IL-10, CCL2, alternative activation of macrophages | CD49+ but not CD49− neutrophils induce resistance to MRSA infection | |||
| Human: | PMN-II: CD11B+, CD49-, TLR2+, TLR4+, TLR7+, TLR9+, ring-form nucleus; MPOlow | Association between CD49d+ neutrophils and allergic disease in humans | |||
| Non atopic patients: 1.625%; atopic patients: 6.57% | |||||
| IL-17+ | Human: ∼70% of circulating neutrophils upon IL-6 and IL-23 stimulation | IL-17A+; IL-17ra+, RORγt+; dectin-2 | ↑ ROS production | Reduction of Aspergillus fumigatus–mediated keratitis | 65 |
| Mouse: ∼17% of bone marrow neutrophils on IL-6 and IL-23 stimulation | ↑ Increase fungal killing capacity | ||||
| LDGs | Human: | Mixed population with cells with band, lobular, or myelocyte-like nuclei | ↓ Phagocytosis, = MPO, =respiratory burst | Promotion of SLE-associated inflammation: induction of IFN-α production by pDCs through NET release | 66,-68 |
| Healthy donors: ∼17% | ↑ IFN-γ; TNF-α | ||||
| SLE patients: 1.2-54% | ↑ Endothelial cell killing capacity | ||||
| ↑ NET production | |||||
| TAN | Not defined | N1 TAN: Met+; hypersegmented nuclei | N1 TAN: ↑ tumor cell killing capacity; ↑ NO; ↑ H2O2; ↑ TNF-α; ↑ ICAM-1; ↓ arg1; ↓ CCL2, ↓CCL5, ↓VEGF; ↓ MMP9 | N1 and N2 TANs inhibit and promotes tumor development, respectively | 69,,-72 |
| N2 TAN: Rounded nuclei | N2 TAN: Rounded nuclei ↓ tumor cell killing capacity; ↓ NO; ↓ H2O2; ↓ TNF-α; ↓ ICAM-1; ↑ arg1; ↑ CCL2, ↑ CCL5, ↑ VEGF; ↑ MMP9 ↑ S100a8; ↑ S100a9; ↑ Prok2; |
| Neutrophil subset . | Prevalence . | Phenotypic properties . | Functional properties . | Homeostatic/pathological relevance . | Reference . |
|---|---|---|---|---|---|
| CD177+ | Human: ∼50% of circulating neutrophils | CD177+ Proteinase 3 | = Apoptosis = transmigration in human peritonitis↓ granule protein mRNA content | CD177 autoantibodies in ANCA-derived vasculitis patients | 41,,,-45 |
| “Aged” | Mouse: | CXCR4highCD62LlowCD11Bhigh CD49high; hypersegmented nucleus; Reduced size and granularity | Regulation of stem cell niche and circadian release of HSPCs to the circulation / Enhanced intravascular inflammation | 46,47 | |
| 55 ± 14% in ZT5 (clearance from the circulation period) | = Apoptosis | ||||
| 8 ± 3% in Z17 (release from the circulation period) | ↑ Phagocytosis and NETosis | ||||
| OLFM4+ | Human: 20-25% of circulating neutrophils | OLFM4 expression in neutrophil-specific granules | = Apoptosis | OLFM4 autoantibodies in ANCA-derived vasculitis patients | 48,,,-52 |
| = Phagocytosis | Enhanced immune response against S aureus and E coli infection in absence of OLFM4 | ||||
| = Transmigration | |||||
| ↓ Cathepsin C activity | |||||
| OLFM4 + NET formation | |||||
| TCR+ | Human: 3-5% of circulating neutrophils | Human: TCRα,β variants | Delayed apoptosis and IL-8 production on CD3/CD28 stimulation | Reduced TCR variants in aged subjects compared to young individuals | 53,54 |
| Mouse: Vα2, Vα5, Vβ1, Vβ16 | |||||
| Angiogenic | Mouse: 2.8% | CD49d+VEGFR1highCXCR4high | ↑MMP9 | Promotes angiogenesis in hypoxic tissue | 55,56 |
| Human: 3.2% | |||||
| CD62Ldim/CD16brightCD62Lbright/CD16dim | Human: 3 hours after LPS administration: | CD62Lbright; CD16bright; multi-lobular nucleus | CD62Ldim/CD16bright: -Inhibition of T-cell proliferation through ROS release and dependent on CD11B expression | Potential implication in sepsis-related immunosuppresion | 57,58 |
| CD62Lbright/CD16bright: 60-70% | CD62Ldim; CD16bright; CD11Chigh; CD11Bhigh; CD54high ; hypersegmented nucleus | ||||
| CD62Ldim/CD16bright:20-25% | CD62Lbright; CD16dim; CD11Clow; CD11Blow; CD54low ; band-form nucleus | ||||
| CD62Lbright/CD16dim:10-15% | |||||
| CD63+ | Human: neutrophils isolated from lung sputum | CD11Bhigh CD66high CD63+CD80+MHC-II+ | ↓ Glutathione activity | Neutrophil elastase-dependent perpetuation of inflammation, infection and progression of cystic fibrosis airway disease | 59,60 |
| ↑ Neutrophil elastase activity | |||||
| ↑ Arg1 | |||||
| IL-13+ | Not defined | Ring-form nucleus; IL-5; IL-13; IL-33; insulin-like growth factor-1; Resistin-like molecule alpha/FIZZ1; chitinase-like 3. | Priming of macrophages towards alternative or M2 phenotype | Accelerated clearance of nematodes by primed macrophages | 61 |
| CD49+ | Mouse: | PMN-I: CD11B−, CD49+, TLR2+, TLR4+, TLR5+, TLR8+; multilobular nucleus, MPOhigh | PMN-I: IL-12, CCL3, classic activation of macrophages | CD49d+ neutrophils promote virus-challenged experimental asthma | 62,-64 |
| Sendai virus infection: 50% of BAL neutrophils | |||||
| MRSA infection: not defined | PMN-II: IL-10, CCL2, alternative activation of macrophages | CD49+ but not CD49− neutrophils induce resistance to MRSA infection | |||
| Human: | PMN-II: CD11B+, CD49-, TLR2+, TLR4+, TLR7+, TLR9+, ring-form nucleus; MPOlow | Association between CD49d+ neutrophils and allergic disease in humans | |||
| Non atopic patients: 1.625%; atopic patients: 6.57% | |||||
| IL-17+ | Human: ∼70% of circulating neutrophils upon IL-6 and IL-23 stimulation | IL-17A+; IL-17ra+, RORγt+; dectin-2 | ↑ ROS production | Reduction of Aspergillus fumigatus–mediated keratitis | 65 |
| Mouse: ∼17% of bone marrow neutrophils on IL-6 and IL-23 stimulation | ↑ Increase fungal killing capacity | ||||
| LDGs | Human: | Mixed population with cells with band, lobular, or myelocyte-like nuclei | ↓ Phagocytosis, = MPO, =respiratory burst | Promotion of SLE-associated inflammation: induction of IFN-α production by pDCs through NET release | 66,-68 |
| Healthy donors: ∼17% | ↑ IFN-γ; TNF-α | ||||
| SLE patients: 1.2-54% | ↑ Endothelial cell killing capacity | ||||
| ↑ NET production | |||||
| TAN | Not defined | N1 TAN: Met+; hypersegmented nuclei | N1 TAN: ↑ tumor cell killing capacity; ↑ NO; ↑ H2O2; ↑ TNF-α; ↑ ICAM-1; ↓ arg1; ↓ CCL2, ↓CCL5, ↓VEGF; ↓ MMP9 | N1 and N2 TANs inhibit and promotes tumor development, respectively | 69,,-72 |
| N2 TAN: Rounded nuclei | N2 TAN: Rounded nuclei ↓ tumor cell killing capacity; ↓ NO; ↓ H2O2; ↓ TNF-α; ↓ ICAM-1; ↑ arg1; ↑ CCL2, ↑ CCL5, ↑ VEGF; ↑ MMP9 ↑ S100a8; ↑ S100a9; ↑ Prok2; |
arg1, arginase 1; BAL, bronchoalveolar lavage; MMP9, metalloproteinase-9; MPO, myeloperoxidase; MRSA, methicillin-resistant Staphylococcus aureus; NO, nitric oxide; pDCs, plasmacytoid dendritic cells; PMN, polymorphonuclear leukocyte; VEGF, vascular endothelial growth factor; ZT, zeitgeber time; ↑, increase; ↓, decrease; =, no change.
Phenotypic and functional adaption by activation
Neutrophil activation by cues derived from sites of inflammation results in exposure of distinct surface markers or de novo synthesis of cytokines, characteristics that are both commonly used to define neutrophil subsets. Neutrophil heterogeneity may thus partly be explained by a differential activation stage of neutrophil subpopulations. After interaction with endothelial selectins and chemotactic molecules neutrophil secretory vesicles are fused with the surface membrane exposing the proteins contained in these vesicles.73-75 In this way, neutrophils activated on the endothelium quickly upregulate CD11b and CD18, thus contributing to firm adhesion.76 In addition, tumor necrosis factor (TNF) receptors located in specific and gelatinase granules are redistributed to the cell membrane after activation.77 Galectin-3 released by macrophages or mast cells is a potent activator of neutrophils. Its receptor, CD66, is stored in specific granules, and stimulation with lipopolysaccharide (LPS)78 or granulocyte colony-stimulating factor (G-CSF)79 induces the secretion of specific granules exposing CD66 and hence primes the neutrophil for a second response to galectin-3. Laminin receptor-1 is located in the specific granules and its binding to the extracellular matrix protein laminin induces the secretion of lysozyme and the production of superoxide, thereby enhancing “second-hit” responses.80 Interestingly, circulating platelets activated by danger signals can also deliver signals through protruding domains presented by a fraction of intravascular neutrophils, a process that promotes neutrophil crawling and recruitment into inflamed tissues.81
Neutrophil subsets in homeostasis
CD177.
In humans, CD177 glycoprotein (NB1) is exclusively expressed on the surface of neutrophils and regulates transmigration across the endothelium through the interaction with platelet endothelial cell adhesion molecule-1.82 In humans, a fraction of CD177+ neutrophils has been identified83 coexpressing membrane-bound proteinase-3,41 a serine protease normally located in primary granules. CD177 expression is required for surface presentation of proteinase-3,84 which in turn facilitates the transmigration of CD177+ neutrophils.85 The variable inter- and intraindividual expression of the CD177–proteinase-3 partnership in circulating neutrophils gained importance when membrane proteinase-3 was identified as an antigen antineutrophil cytoplasmic antibody (ANCA)-dependent vasculitis.86 Accordingly, the levels of neutrophils coexpressing proteinase-3 and CD177 are augmented in patients of ANCA-dependent vasculitis87 and associate with increased risk of relapse.88 However, the direct implication of CD177+ or CD177− subsets in ANCA-derived vasculitis and other inflammatory diseases remains unknown. To understand the potential role of CD177, a mouse genetically deficient in this protein was generated.89 As in humans,82 the absence of CD177 did not affect the migratory capacity of neutrophils; however, it caused cell death in this mouse model.89 In conclusion, although the frequency of CD177+ neutrophils during inflammatory disease is consistently augmented, the functional properties of this neutrophil subset are controversial and will require further investigation.
Aged neutrophils.
Neutrophil numbers are controlled by a fine balance between production, retention, mobilization, margination, and clearance. Neutrophil retention in the bone marrow is regulated by the coordinated action of CXCL12 and its receptor CXCR4, whereas neutrophil egress depends on CXCR2 and its ligands.90,91 CXCR4 is expressed at low levels in bone marrow–resident neutrophils while almost undetectable on the surface of circulating neutrophils.92 Interestingly, culture of human neutrophils for 20 hours triggers the upregulation of CXCR4 expression and increases their migratory capacity toward CXCL12 and the predilection to home to the bone marrow where they are cleared.93 In mice, this subpopulation of neutrophils, named “senescent” or “aged” neutrophils, are characterized by the upregulation of CXCR4 and CD11b and the loss of CD62L, size reduction, and hypersegmentation of the nucleus.46 Interestingly, clearance of aged neutrophils by bone marrow–resident macrophages downregulated CXCL12 production by stromal cells and induced the mobilization of hematopoietic stem progenitor cells (HSPCs) to the circulation.46 Moreover, numbers of circulating aged neutrophils and their clearance in the bone marrow fluctuated during the day, a process that controlled the circadian-oscillations of HSPC numbers in the blood.46 Besides this noninflammatory function, aging predisposes overactivation of neutrophils, which increases CD11b expression and susceptibility of neutrophil extracellular trap (NET) formation.47 Furthermore, this recent study provides evidence that neutrophil ageing is regulated by the microbiota in a TLR-dependent fashion. Interestingly, this aged subpopulation predominates in mice and humans under pathological conditions and dramatically worsens the disease outcome in a mouse model of sickle cell disease.47 Overall, these findings illustrate that aging may be a source of neutrophil heterogeneity that predisposes to functional changes with implications in homeostasis and inflammation.
Olfactomedin-4.
Olfactomedin-4 (OLFM4) is present in neutrophil-specific granules of 20% to 25% of human circulating neutrophils.48 Although the specific function of this protein in neutrophils is still unknown, neutrophil-derived OLFM4 has been suggested to induce autoimmune responses (supplemental Discussion 4).
T-cell receptor.
A repertoire of T-cell receptor (TCR) variants has unexpectedly been detected in a subset of circulating human neutrophils.53 A detailed discussion on this subset can be found in the supplemental Discussion 5.
Angiogenic neutrophils.
Under ischemia conditions, a distinct subset of neutrophils is recruited to hypoxic areas with unique phenotypic and angiogenic properties that facilitate restoration of the oxygen supply in the affected tissue55 (supplemental Discussion 6).
Neutrophil subsets in pathologic conditions
Pathogen-mediated systemic inflammation.
Systemic inflammation elicited by injury or sepsis causes overproduction and hyperreaction of neutrophils. After the initial immune response, nonresolved inflammation results in a prolonged action of neutrophils, which may cause collateral damage.94 However, it is during this latter phase when an anti-inflammatory response is produced, resulting in immunosuppression and increasing the risk for mortality.95 Because neutrophilia is still occurring during this phase of the disease, Pillay and colleagues aimed to examine specific alterations in the phenotype and function of neutrophils that may explain the observed immunosuppression.57 Analysis of circulating neutrophils in LPS-challenged healthy volunteers identified fully competent mature CD16bright neutrophils that were accompanied by the release of banded CD16dim neutrophils with increased reactive oxygen species (ROS) production but impaired antimicrobial function.57 Consequent analysis of LPS-driven neutrophil heterogeneity by the same group revealed the existence of a novel subset of mature, hypersegmented human neutrophils with immunosuppressive activity.58 This subset was defined by the surface markers CD11cbrightCD62LdimCD11bbrightCD16bright and was able to suppress T-cell proliferation through the release of ROS and required the expression of CD11b. The ability to suppress T-cell proliferation and responses has also been described for myeloid-derived suppressor cells (MDSCs), a leukocytic population identified in different pathologies such as infection or cancer.96 This MDSC population is composed of a monocytic and granulocytic subpopulation with the ability to suppress T-cell proliferation and activation in a ROS- and Arginase I–dependent fashion. Although the granulocytic counterparts of this population (G-MDSC) exhibit phenotypic and functional differences compared with those observed by Pillay and colleagues,96 both share this immunosuppressive activity. The lack of a consensus when phenotyping G-MDSCs96 makes it difficult to discern whether these 2 populations belong to a unique heterogeneous population with immunosuppressive abilities.
In pathogen-mediated inflammation, a number of other neutrophil subsets have been described. Refer to the supplemental Discussion for information on CD63+, IL-13+, CD49+, and IL-17+ neutrophil subsets (supplemental Discussions 1, 2, 3, and 7).
Systemic lupus erythematosus.
Initially an abnormal population of neutrophils isolated from patients of systemic lupus erythematosus (SLE) and other autoimmune disorders was characterized by displaying different density properties.97 The functional characterization of this subpopulation of neutrophils, referred to as low-density neutrophils (LDGs), revealed a proinflammatory, activated phenotype with exacerbated production of type I interferons (IFNs) and reduced phagocytic capacity.66 Human LDGs were also shown to be highly susceptible to form NETs in the absence of stimulation, which was responsible for the enhanced endothelial cell (EC) toxicity displayed by this subset.67 Indeed, anti-ribonucleoprotein antibodies developed in SLE patients induced NET release by neutrophils causing a potent activation of dendritic cells and production of IFN-α.68,98 Self-DNA and antimicrobial peptides present in the NET structures led to the generation of autoantibodies triggering a positive feedback loop that amplified the inflammatory response. Of note, LDGs have also been identified in other physiological or pathogenic immune alterations such as cancer,99 sepsis,100 pregnancy,101 diabetes,102 or HIV infection.103 Interestingly, these LDG subpopulations share the ability to suppress the immune response, a characteristic that contrasts the proinflammatory phenotype of SLE-associated neutrophils.
Cancer.
During tumor-driven inflammation, ≥2 different subpopulations of neutrophils have been described that exert antagonist functions. Fridlender et al demonstrated in mice that tumors were infiltrated by tumor-associated neutrophils (TANs) characterized by a hypersegmented nucleus, increased production of proinflammatory cytokines, and enhanced tumor cell killing capacity.69 In contrast to this antitumoral population, referred to as N1, an alternative population of TANs (N2) was identified that displayed an immature phenotype and increased arginase activity and promoted tumor growth. TANs exhibit clear phenotypic and functional parallels with M1 and M2 macrophage polarization patterns. For instance, N2 neutrophils and M2 macrophages, both of which favor tumor growth, share elevated arginase activity, which is responsible for inhibition of T-cell proliferation by depletion of l-arginine.104 The “polarization” of TANs from a protumoral toward an antitumoral phenotype was attributed to the action of the anti-inflammatory cytokine transforming growth factor (TGF)-β.69 Interesting in this context is that the primary tumor hijacked the IL-17/G-CSF axis to promote the mobilization of protumoral neutrophils.70 It is presently unclear whether these reported populations of protumoral neutrophils share a common origin and function. On the other hand, IFN-β has been involved in the activation of mouse and human neutrophils toward an antitumor phenotype, as demonstrated by genetically deficient mouse models71,105 and IFN therapies.105 Similarly, Finisguerra and colleagues recently demonstrated the importance of Met, a proto-oncogene whose expression in mouse and human neutrophils is essential in the transmigration, cytotoxic, and protumoral capacity of these cells.72
As mentioned before, MDSCs are a separate population of cells with monocytic or granulocytic phenotypes that appear in tumor-bearing mice and cancer patients and are characterized by their ability to suppress T-cell proliferation and activity.96 Transcriptomic analysis of TANs compared with G-MDSCs and naïve neutrophils suggested that these 3 populations are distinct, with a surprisingly close proximity between G-MDSCs and naïve neutrophils compared with the TAN subpopulation.106 Hence, it is conceivable that the N1 antitumoral and N2 protumoral phenotypes described by Fridlender and others originated from naïve neutrophils or G-MDSCs, respectively. However, because naïve neutrophils were isolated from tumor-free mice, a more detailed study would be required to define the functional differences between suppressive (G-MDSCs) or nonsuppressive (naïve) neutrophils isolated from tumor-bearing mice. Unfortunately, the only method to reliably distinguish G-MDSCs from normal neutrophils depends on the analysis of their suppressive capacity, a technical handicap that impedes the proper isolation of both populations without compromising their function during manipulation. In this regard, advantage should be taken of the density properties attributed to G-MDSCs that resemble those exhibited by LDGs. Sagiv et al demonstrated that tumor-associated mouse and human LDGs are a heterogeneous population composed of mature and immature neutrophils with suppressive capacity.99 Interestingly, the mature members of this population originated from high-density neutrophils, which acquire immunosuppressive functions after exposure to TGF-β. Thus, this study provides a plausible explanation as to the origin of suppressive, mature neutrophils (N2 TAN). On the other hand, the authors suggested that the immature LDGs originate from immature neutrophils that are released from the bone marrow before completing their maturation. Because LDGs isolated from SLE patients do not exhibit immunosuppressive capacity, it is reasonable to considerer that the acquisition of low-density properties and the immunosuppressive capacity are independent processes that occur after neutrophil activation to thus far unknown environmental signals. Interestingly, in vitro activation of circulating neutrophils isolated from healthy donors induced the acquisition of the suppressive capacity and low-density properties probably after degranulation.107 In line with these results, LDGs101,103 and G-MDSCs107 express high levels of CD66b, a receptor mobilized from the granules to the surface on cell activation. However, additional studies are needed to further clarify the exact origin of these cells and the external signals that confer their immunosuppressive capacities.
Conclusions
As technical handicaps are being solved by the generation of novel techniques and animal models, a wide range of new and fascinating functions attributed to neutrophils is being discovered. The classical view of neutrophils does not accommodate all aspects of neutrophil biology, and thus the previously ascribed functions of neutrophils seem incomplete. The recently described prolonged lifespan of neutrophils, their ability to de novo synthesize cytokines, and the capacity to recirculate through different tissues and organs extend their repertoire of immunomodulatory functions by interacting and modulating the immune response exerted by both the innate and adaptive system. Moreover, these recently identified properties of neutrophils align with the increasing evidence for the existence of neutrophil subpopulations with distinct phenotypic and functional characteristics. It is indeed this renovated view of neutrophils as a plastic cell that may partly explain neutrophil heterogeneity. Understanding neutrophil heterogeneity may be instrumental to develop novel therapies that specifically target pathogenic neutrophil subsets without compromising immunity.
The online version of this article contains a data supplement.
Acknowledgments
O.S. is supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (VIDI project 91712303), the Academic Medical Center Research Council, the Deutsche Forschungsgemeinschaft (SO876/6-1, SFB914-B08, SFB1123-A06/B05), and the Ludwig Maximilian University excellent program. C.S.-R. is supported by the FöFoLe program of the LMU Medical Faculty. A.H. is supported by SAF2012-31142 from the Ministry of Economy and Competitiveness. The Centro Nacional de Investigaciones Cardiovasculares Carlos III is supported by the Ministry of Economy and Competitiveness and the Pro-CNIC Foundation and is a Severo Ochoa Center of Excellence (Ministry of Economy and Competitiveness award SEV-2015-0505).
Authorship
Contribution: C.S.-R. wrote the manuscript; and A.H. and O.S. contributed to manuscript preparation and revision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlos Silvestre-Roig, Institute for Cardiovascular Prevention, LMU Munich, Pettenkoferstrasse 9, 80336 Munich, Germany; e-mail: carlos.silvestre@med.uni-muenchen.de; or Oliver Soehnlein, Institute for Cardiovascular Prevention, LMU Munich, Pettenkoferstrasse 9, 80336 Munich, Germany; e-mail: oliver.soehnlein@gmail.com.