In this issue of Blood, McGowan et al show that institution-wide replacement of unfractionated heparin (UFH) with low-molecular-weight heparin (LMWH) substantially reduces the burden of heparin-induced thrombocytopenia (HIT).1 Because it is well-established that LMWH is less likely to cause this highly prothrombotic adverse drug reaction than UFH,2 it seems intuitive, at first, that using less UFH would reduce the incidence of HIT. However, implementing this policy at a system-wide level is far from simple. The in-hospital unit cost of LMWH is 6- to 8-fold higher than that of UFH (average, $3 vs $24 per day in 2013 US dollars)3 and, in an age where providing venous thromboprophylaxis is mandatory for hospitalized patients, choosing UFH is like picking low-hanging fruit for cost-conscious institutions. The absence of compelling data showing that LMWH is more effective than UFH for thromboprophylaxis or treatment does nothing to discourage this practice.4,5
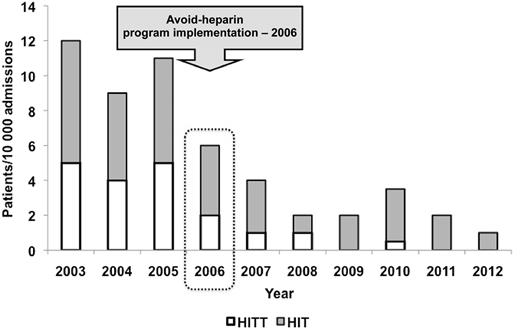
Annual incidence of HIT/HIT with thrombosis (HITT) per 10 000 admissions. See Figure 2 in the article by McGowan et al that begins on page 1954.
Annual incidence of HIT/HIT with thrombosis (HITT) per 10 000 admissions. See Figure 2 in the article by McGowan et al that begins on page 1954.
In their before-and-after analysis, McGowan et al implemented an Avoid-Heparin Initiative at a tertiary care center that involved replacing UFH with LMWH for all prophylactic and therapeutic indications except during cardiac surgery or hemodialysis and in selected acute coronary syndrome patients.1 Importantly, all heparin flushes for arterial and central venous lines were replaced with saline. When all cases of suspected HIT were adjudicated, they found that the annual rate of suspected HIT after the initiative decreased by 42%, adjudicated HIT decreased by 79%, and HIT with thrombosis decreased by 91% (rates per 10 000 admissions given in figure). Furthermore, the savings in HIT-related costs for the institution were substantial (>$250 000 per year CAN).
This study is one of few in the literature that focuses on prevention of HIT rather than diagnosis or treatment. Greinacher et al performed a similar before-and-after analysis when enoxaparin replaced UFH for thromboprophylaxis following hip or knee replacement surgery at their institutions.6 These investigators also found that patients who received LMWH had a significantly lower frequency of HIT compared with patients who received UFH (0%; 95% confidence interval, 0.0-1.4 vs 5.2%; 95% confidence interval, 2.7-8.9; P < .001). Others have shown similar results using decision-analysis modeling7,8 ; however, the study by McGowan et al is the first to demonstrate the impact of large-scale removal of heparin from clinical practice in the real world.
We can gain further insight into the benefits of primary prevention of HIT from a cost-effectiveness analysis of a randomized controlled trial that compared LMWH with UFH for thromboprophylaxis for critically ill patients.3 In the Prophylaxis for Thromboembolism in Critical Care Trial, 3764 patients who were admitted to the intensive care unit were randomized to either UFH or dalteparin during their stay in the unit.9 There was no statistical difference in the primary outcome measure, which was the incidence of proximal deep vein thrombosis. However, dalteparin was found to be more cost-effective than UFH in a substudy (n = 2344) that was run concurrently with the main study.3 The authors attributed the difference in cost to a lower rate of HIT (0.3% vs 0.6%) and pulmonary embolism (1.3% vs 2.3%) in the dalteparin arm with a corresponding overall lower use of resources. To put these findings into context, the authors state that “if an [intensive care unit] with 1000 medical-surgical admissions per year used UFH instead of LMWH for prevention of [venous thromboembolism], the annual incremental cost would be $1 000 000-$1 500 000 with similar or worse clinical outcomes.”3
So, if LMWH reduces both the clinical and financial burdens of HIT, is it time to remove UFH completely from the drug formularies at our institutions? The answer is no. Heparin is still the best option for patients who are undergoing cardiovascular surgery: those with renal failure and those at high risk for bleeding that require a rapid reversal agent. Furthermore, these indications are likely to remain even as the direct oral anticoagulants take on more of a role in the prevention and treatment of thromboembolism. For now, McGowan et al should be congratulated on a study that eloquently shows that when you take into consideration the costs of suspected and confirmed HIT, UFH is not the bargain it first appears to be.
Conflict-of-interest disclosure: The author has received lecture honoraria from Pfizer and research funding from Bayer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal