The transcription factor signal transducer and activator of transcription 3 (STAT3) is perhaps best known for its prosurvival effects in a wide variety of cancers, but for some, including acute myeloid leukemia (AML), its role in immune evasion may be just as important. In this issue of Blood, Zhang et al1 report the development of an engineered STAT3 decoy oligodeoxynucleotide (dODN) that is stable in serum, is taken up specifically by target cells, and exerts its antileukemia effects largely by restoring the host anti-AML immune response.
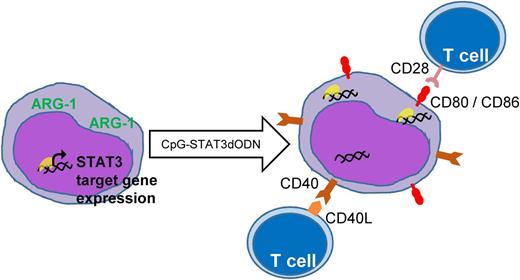
The STAT3dODN sequesters STAT3 transcription factors in the cytoplasm, thereby reducing expression of oncogenic STAT3 target genes. Additionally, treatment with the dODN results in increased expression of costimulatory molecules that recruit T cells and activate an effective anti-AML immune response.
The STAT3dODN sequesters STAT3 transcription factors in the cytoplasm, thereby reducing expression of oncogenic STAT3 target genes. Additionally, treatment with the dODN results in increased expression of costimulatory molecules that recruit T cells and activate an effective anti-AML immune response.
Since the declaration of STAT3 as an oncogene by Bromberg et al in 1999,2 the rationale for blocking its activity as a therapeutic strategy has been building. STAT3 target genes promote survival, proliferation, angiogenesis, metastasis, and chemotherapy resistance (reviewed by Carpenter and Lo3 ). More recently, we have added immune evasion to this list of STAT3-driven oncogenic processes, in large part due to the work of Kortylewski and colleagues.4-6
Although the rationale for blocking STAT3 is straightforward, the means to do so is not. Like other transcription factors, the STAT3 protein structure does not offer an obviously druggable site analogous to, for example, the adenosine triphosphate binding pocket of a kinase. Nevertheless, a number of laboratories have reported small molecule tool compounds with anticancer activity in preclinical assays.7-10 Such inhibitors generally have potencies in the low micromolar range and lack specificity for STAT3 over other STAT proteins. Another approach to blocking STAT3 activity has been with dODNs that are based on the consensus promoter sequence that STAT3 recognizes. dODNs work by binding activated STAT3 dimers and sequestering them in the cytoplasm. However, naked ODNs do not enter cells well, and they are rapidly degraded by serum nucleases, so their usefulness in vivo has been limited.
Zhang et al designed a clever system to turn the STAT3dODN into a viable therapeutic molecule. First, they conjugated the dODN to a cytosine guanine dinucleotide (CpG)-containing sequence to facilitate its uptake by cells expressing Toll-like receptor 9 (TLR9). TLR9 is normally expressed by antigen-presenting cells, such as B cells, macrophages, and dendritic cells. Importantly, TLR9 is highly expressed by most AML cells and not expressed by normal hematopoietic stem and progenitor cells.1 They first reported the success of this method in 2014, using a CpG–small interfering RNA conjugate targeting STAT3.5 In the current paper, they further improved the strategy by chemically modifying the CpG-ODN to resist degradation by serum nucleases, thereby generating a molecule with a clinically acceptable half-life.
Another advance reported by Zhang et al comes from the biology they learned with this STAT3dODN. In a series of elegant experiments comparing the effects of the decoy in vitro, in an immunocompromised xenograft AML model, and in an immunocompetent mouse AML model, Zhang et al show that the dODN works through direct cytotoxicity to AML cells and through indirect enhancement of the host immune response. Specifically, they show that blocking STAT3 activity in AML cells leads to increased costimulatory molecule expression, thus making them better T-cell activators. In other words, STAT3 activity allows AML cells to hide from T cells, and blocking STAT3 blows their cover.
As we envision this strategy in a clinical trial, one wonders what adverse effects might be seen in patients. In that regard, it is important to understand that, unlike systemic treatment with a small molecule STAT3 inhibitor, uptake of the dODN is targeted to AML blasts and some immune cells. Hematopoietic stem cells are spared, as are T cells: the effectors of the second prong of the dODN-induced antileukemia response. Therefore, myelosuppression is less of an immediate concern, although abnormal inflammatory responses might be possible. Another consideration is in what clinical setting this therapeutic strategy would be most beneficial. Currently, immunotherapies (such as modified T cells targeting tumor antigens) are generally used in the setting of minimal residual disease, although some patients with high leukemia disease burden have been successfully treated this way. It remains to be seen how this kind of immune reactivation would work in combination with other more traditional leukemia therapies. Because the treatment options for AML are so limited, and the cures after relapse are so rare, novel ideas like this one hold great promise.
Conflict-of-interest disclosure: The author declares no competing financial interests.