In this issue of Blood, Welsh and colleagues determine how platelet thrombi limit the loss of plasma-borne proteins from the microvasculature.1
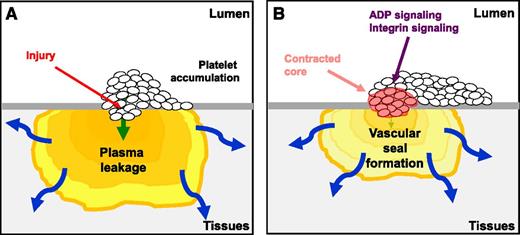
The accumulation of platelets at the site of penetrating vascular injury forms a seal to prevent the loss of plasma proteins. (A) The initial recruitment of platelets stems the loss of blood cells and begins to reduce plasma leakage. (B) The prevention of vascular leakage is enhanced by intrathrombus remodeling through ADP and integrin αIIbβ3 signaling resulting in formation of a contracted thrombus core. The figure has been adapted from Figure 7 in the article by Welsh et al that begins on page 1598.
The accumulation of platelets at the site of penetrating vascular injury forms a seal to prevent the loss of plasma proteins. (A) The initial recruitment of platelets stems the loss of blood cells and begins to reduce plasma leakage. (B) The prevention of vascular leakage is enhanced by intrathrombus remodeling through ADP and integrin αIIbβ3 signaling resulting in formation of a contracted thrombus core. The figure has been adapted from Figure 7 in the article by Welsh et al that begins on page 1598.
The concept that platelets prevent leakage from blood vessels following a penetrating injury will come as little surprise to most readers, but exactly how platelets physically contribute to this process is not well understood. Such injuries result in the loss of blood cells and plasma into the surrounding tissues, and both forms of loss must be halted quickly. Platelets are believed to form the first line of defense in such situations, forming a thrombus that is capable of plugging the hole and supplying molecules that contribute to localized inflammation and wound healing.
In recent years, innovations in intravital microscopy have provided insight into the control mechanisms that allow platelet thrombi to form within the microcirculation of mice. Such approaches allow the accumulation of platelets at sites of vascular injury to be visualized and quantified, enabling the roles of specific proteins to be assessed, exploiting transgenic mice, selective inhibitors, or pharmacologic agents.2 A range of injury models has been developed for intravital analysis including mechanical, chemical, photochemical, and electrical injury, and a model that has gained extensive use is focal injury using a laser.3
In a recent series of studies, the architecture of thrombi formed in mice has been explored.4,5 Thrombi formed in cremaster muscle arterioles possess a zoned structure, with a tightly packed inner core of platelets which results from outside-in signaling through integrin αIIbβ3, pulling platelets closer together.6,7 Secondary platelet agonists emanating from activated platelets on the edge of the core cause the accumulation of platelets that form a less densely packed shell layer. A consequence of the tightly packed core is restricted permeation of plasma-borne molecules through the core, and therefore greater local thrombin activity resulting in fibrin formation and increased platelet activation.6-9 Indeed, inhibition of thrombin disrupts thrombus core structure, while the size of the thrombus shell is reduced in the presence of adenosine 5′-diphosphate (ADP) receptor antagonists.6
In this issue, Welsh et al have explored which features of the heterogeneous architecture of platelet thrombi explain their ability to seal blood vessels to achieve “plasma stasis,” and determined whether the use of antiplatelet and anticoagulant agents which impair thrombus structure interfere with the formation of a vascular seal.1 To achieve this goal an elegant method was developed in which albumin conjugated to caged fluorophore was infused into mice. Repeated uncaging of the sensor with light in the vicinity of thrombi formed following penetrating laser injury allowed extravascular accumulation and dispersion of plasma proteins to be measured in real time.
Thrombus formation was studied in cremaster muscle venules, in which the heterogeneous thrombus structure previously seen in arterioles was also observed. Although cessation of red blood cell leakage occurred within seconds of injury, plasma protein leakage continued over a period of minutes, although dramatic reductions in this occurred during the first 3 minutes, beyond the point of maximum platelet and fibrin accumulation, suggesting that structural changes within the thrombus may control the leakage of plasma. Indeed, infusion of eptifibatide to block integrin αIIbβ3, which reduced platelet accumulation but had no effect on peak fibrin production or red cell loss, resulted in increased albumin leakage. Interestingly, increasing the recruitment of platelets to thrombi in transgenic mice expressing mutated Gi2α did not enhance vessel sealing, suggesting that thrombus size alone did not explain the hemostatic potential of a thrombus.
These observations led the team to establish whether defective platelet retraction altered vessel leakiness. This was studied in transgenic mice in which integrin αIIbβ3 is incapable of outside-in signaling.10 Plasma leakage was extended following initial reductions as thrombi formed. This suggests that platelet recruitment controls initial reductions in plasma leakage, with platelet retraction and resultant intrathrombus remodeling further reducing loss of plasma (see figure).
So how do these observations relate to the zoned structures of thrombi? One clue comes from the ability of eptifibatide to greatly increase leakage of labeled albumin, while peak fibrin levels were unaltered, which may suggest, surprisingly, that plasma stasis is not dependent on fibrin. This was tested through infusion of the thrombin antagonist hirudin, which abolished fibrin accumulation and reduced thrombus size, but vessel sealing was unaffected. Conversely, infusion of an ADP receptor antagonist that resulted in a reduction in the size of the P-selectin–negative shell was associated with increased plasma protein extravasation. This indicates a key role for ADP, while suggesting that α-granule secretion (noted by P-selectin exposure) is not directly linked to tight vascular sealing.
Further scrutiny of labeled albumin extravasation and dispersion rates revealed that in conditions (or transgenic mice) where plasma leakage was enhanced, accumulation rates exceeded dispersion rates. Therefore, changes in thrombus architecture (for example, following exposure to ADP receptor antagonists) that upset the likelihood of extravasation rates falling below dispersion result in increased buildup of proteins in surrounding tissues.
Previous studies have indicated that the zoned structure of a platelet thrombus is required to enable localized accumulation of different agonists within the thrombus, which in turn reinforce this structure through contraction of the core and the formation of a gradient of ADP and thromboxane A2 allowing shell growth. This current study indicates the potential importance of distinctive intrathrombus zones because the prevention of the loss of plasma proteins requires platelet accumulation, ADP-dependent platelet signaling, and platelet contraction. It will be important to determine whether the phenomena discovered in this study are observed following other forms of vascular injury, noting that some variability in leakage measurements in the present study may have been due to different extents of injury. Further work will also be needed to determine whether similar properties of platelet thrombi are observed in arterioles and in the macrocirculation.
This study provides new insight into the molecular and physiological processes that ensure that blood vessel leakage is prevented following penetrating injury and indicates how antiplatelet agents may delay the formation of an effective vascular seal.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal