Abstract
Thrombocytopenia is defined as a status in which platelet numbers are reduced. Imbalance between the homeostatic regulation of platelet generation and destruction is 1 potential cause of thrombocytopenia. In adults, platelet generation is a 2-stage process entailing the differentiation of hematopoietic stem cells into mature megakaryocytes (MKs; known as megakaryopoiesis) and release of platelets from MKs (known as thrombopoiesis or platelet biogenesis). Until recently, information about the genetic defects responsible for congenital thrombocytopenia was only available for a few forms of the disease. However, investigations over the past 15 years have identified mutations in genes encoding >20 different proteins that are responsible for these disorders, which has advanced our understanding of megakaryopoiesis and thrombopoiesis. The underlying pathogenic mechanisms can be categorized as (1) defects in MK lineage commitment and differentiation, (2) defects in MK maturation, and (3) defect in platelet release. Using these developmental stage categories, we here update recently described mechanisms underlying megakaryopoiesis and thrombopoiesis and discuss the association between platelet generation systems and thrombocytopenia.
New standing of megakaryocytes in hematopoiesis
Within the adult bone marrow (BM), hematopoietic stem cells (HSCs) are maintained for their multipotency and self-renewal capability, including the osteoblastic niche and/or vascular niche and others. Dysregulation of HSCs or the niche system, perhaps due to augmented immunity, epigenetic deficiency, or impaired transcriptional regulation1,2 can disrupt all known blood cell production and maintenance. Moreover, diminished HSC self-renewal and/or multipotential differentiation contributes to thrombocytopenia and the development of trilineage BM failure.
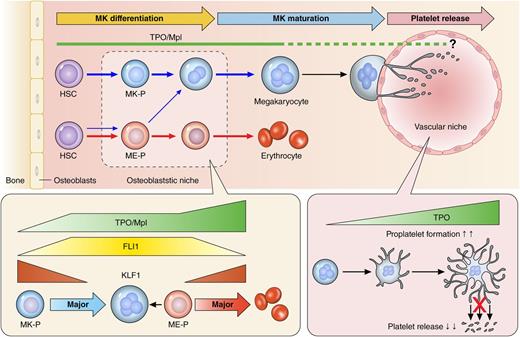
Megakaryocytes (MKs) migrate from the HSC osteoblastic niche to the vascular niche (Figure 1), where they mature and eventually extend proplatelets and release platelets into the bloodstream.3 The hematopoietic hierarchy from HSCs is spatially and temporally regulated by transcription factors, signaling and adhesion molecules, and cytokines and chemokines. Among these many mediators, the most important for megakaryopoiesis is thrombopoietin (TPO), the ligand for the c-Mpl receptor.4 The importance of TPO and c-Mpl is evidenced by the fact that the first report of in vitro platelet generation was published soon after their discovery.5 Moreover, congenital MPL deficiency in humans or knockout of Mpl or Thpo in mice manifests as severe thrombocytopenia.6,7
A revised model in mouse and human assumes preferential MK lineage commitment and differentiation through a pathway beginning from HSC and passing MK-P in the presence of TPO/Mpl signaling.23,23 In contrast, ME-Ps likely contribute to erythrocyte production.25 During megakaryopoiesis and erythropoiesis, TPO/Mpl signaling controls expression of the transcriptional factors Friend leukemia virus integration 1 (FLI1) and Kruppel-like factor 1 (KLF1), downstream of c-Mpl, which in turn regulates the individual blood cell types.25 On the other hand, a recent new mechanism of platelet release from MKs by IL-1α and TPO demonstrated that an excess of TPO during platelet biogenesis appears to inhibit platelet release.49
A revised model in mouse and human assumes preferential MK lineage commitment and differentiation through a pathway beginning from HSC and passing MK-P in the presence of TPO/Mpl signaling.23,23 In contrast, ME-Ps likely contribute to erythrocyte production.25 During megakaryopoiesis and erythropoiesis, TPO/Mpl signaling controls expression of the transcriptional factors Friend leukemia virus integration 1 (FLI1) and Kruppel-like factor 1 (KLF1), downstream of c-Mpl, which in turn regulates the individual blood cell types.25 On the other hand, a recent new mechanism of platelet release from MKs by IL-1α and TPO demonstrated that an excess of TPO during platelet biogenesis appears to inhibit platelet release.49
Extensive investigation has revealed TPO to be indispensable for MK growth and differentiation and, in turn, maintenance of normal peripheral blood platelet levels.8,9 However, the exact mechanisms by which TPO regulates the maintenance of HSCs, their differentiation into MKs, MK maturation, and subsequent platelet release are unclear. In a recent study, Hoffmeister’s group elegantly showed how abnormal platelets in the circulation influence the Janus kinase 2/signal transducer and activator of transcription signaling axis in the liver, leading to the upregulation of TPO production10 ; this observation diverges from the sponge theory (regulation of circulating TPO levels by platelet c-Mpl–mediated adsorption) for TPO production.11 Interestingly, using a platelet factor 4 (PF4)-Cre system,12 Ng et al showed that TPO is not indispensable for the final stage of MK maturation and subsequent platelet biogenesis.9
In the classical model of hematopoiesis starting from HSCs at the apex, the lymphoid (common lymphoid progenitors) and myeloid (common myeloid progenitors [CM-Ps]) pathways are initially separate. CM-Ps further divide into megakaryocyte-erythrocyte bipotent progenitors (ME-Ps) and other myeloid cells.13 In humans, the ME-P population expresses the CD41a+CD235a+ phenotype.14 However, these bifurcations bring with them a substantial risk of heterogeneous populations when deriving MKs and erythrocytes. Not surprisingly, later studies demonstrated that the classical model is overly simplistic, and hematopoiesis has now been deconstructed into 2 sequential phases: primitive hematopoiesis and definitive hematopoiesis. HSCs emerge through definitive hematopoiesis, which occurs at embryonic day 10.5 (E10.5) in mice, 3 days after primitive hematopoiesis. The primitive hematopoiesis phase occurs within the yolk sac and generates nucleated erythrocytes, erythroblasts, progenitors of definitive erythrocytes (or definitive type erythroblasts), and MKs. Definitive hematopoiesis occurs in multiple regions, but the HSCs converge in the fetal liver, where they expand before settling at their final destination.15 In addition, results from several studies indicate that an endothelial-hematopoietic transition is crucial for hematopoiesis to occur.16 These endothelial cells are referred to as hemogenic endothelium and are recognized by their expression of the transcription factor runt-related transcription factor 1 (Runx1).17 MKs have been observed during both primitive and definitive hematopoiesis, but with different ploidy: whereas MKs are only diploid during primitive hematopoiesis and unlikely to be the primary source of platelets in adults,18 mature MKs are polyploid in adults.
The findings outlined in the previous paragraphs suggest that the different hematopoietic lineages cannot be easily partitioned. Doulatov et al showed, for example, that myeloid lineages can appear in cell populations that should, according to the classical model, be committed lymphoid.19 More interestingly, Morita et al showed that HSCs can themselves be divided into subpopulations with different potentials for the erythrocyte and MK lineages.20 Even more surprising reports suggest that MKs are an HSC niche in murine BM. Although individual reports have proposed different molecular mechanisms for the maintenance of HSCs in a quiescent state (ie, via PF4 [CXC motif ligand 4 (CXCL4)], tumor growth factor-β, or TPO),1,21 for MKs to be an HSC niche, MK emergence would be expected to occur directly from HSCs (Figure 1 upper illustration). Consistent with this idea, Sanjuan-Pla et al demonstrated that platelet-biased von Willebrand factor (vWF)-expressing HSCs are present among a heterogeneous population of murine BM HSCs.22 Shortly before that work, Yamamoto et al reported the myeloid bypass model, which consists of direct conversion of MK progenitors (MK-Ps) and ME-Ps or CM-Ps, all of which show repopulation (self-renewal) potential (note that MK-P is directly generated from HSC in the upper illustration in Figure 1).23 In addition, Nishikii et al very recently showed that a murine glycoprotein Ibα (GPIbα; CD42b)-expressing HSC population can give rise directly to self-replicating MK-Ps.24 Although these reports provided only limited experimental evidence for differentiation into the MK lineage after BM transplantation, collectively they strongly indicate that HSCs and MKs are in very close proximity. This new bypass pathway from HSCs to MK-Ps begs the question of what is the role of ME-Ps.
New role for TPO/Mpl signaling in megakaryopoiesis and thrombopoiesis
TPO/Mpl signaling is indispensable for MK growth and differentiation and for HSC maintenance, and defective expression of MPL causes congenital amegakaryocytic thrombocytopenia (CAMT).6 CAMT is an autosomal-recessive disorder that presents itself at birth with isolated thrombocytopenia and progresses to aplastic anemia and trilineage BM failure. At present, the only cure is HSC transplantation. Although Mpl knockout mice exhibit severe thrombocytopenia, the erythroid and myeloid lineages remain intact throughout life, indicating that the dependency of HSCs on TPO/Mpl signaling differs between humans and mice.7
Using induced pluripotent stem cells generated from skin fibroblasts collected from a CAMT patient who received HSC transplantation therapy,6 Hirata et al recently elucidated the roles of human TPO/Mpl signaling.25 They demonstrated that (1) human TPO/Mpl signaling is indispensable for conversion from HSCs/multipotent progenitors to ME-Ps, which accounts for the anemia manifested by CAMT patients (erythrocyte defect) and (2) enhanced TPO/Mpl signaling promotes erythropoiesis but not megakaryopoiesis through preferential inhibition of FLI1 and subsequent activation of the erythroid master regulator KLF1 (Figure 1 lower illustration). The cross-antagonism of KLF1 toward erythropoiesis and FLI1 toward megakaryopoiesis has been proposed as a means for bidirectional control of lineage fate at the ME-P bifurcation,26 which results in ME-Ps contributing primarily to the red blood cell supply and not to platelets. On the other hand, Kuvardina et al reported that during megakaryopoiesis, Runx1 promotes epigenetic repression of KLF1,27 though it is also known that Bmi1, a core component of the polycomb-repressive complex 1 (PRC1), binds directly to the Runx1/core-binding factor β (CBFβ) transcription factor complex, thereby promoting both MK differentiation and maturation.28 These findings led to a hypothesis where a Bmi1/Runx1-mediated mechanism that directly converts HSCs to MK-Ps enables the generation of large numbers of platelets under the control of TPO/Mpl signaling. Accordingly, Nakamura et al used human induced pluripotent stem cells to establish artificial MK-Ps, which through regulation of the Bmi1/Runx1 cascade were able to mature and then shed functional platelets.29
New players in thrombopoiesis
Platelets are the end products of membrane protrusions from MKs that extend into the sinusoidal vessels, where they are sheered off by blood flow. A single MK will generate thousands of platelets before its membrane is exhausted, after which the remaining cell body is degraded. To produce such a large number of platelets, MKs must undergo extraordinary amounts of membrane invagination-related structural changes.30 Endomitosis is the phenomenon that increases the ploidy, cell volume, and surface area-to-volume ratio of MKs, and accounts for the additional membrane necessary for platelet construction.
The next step in platelet production involves cytoskeletally regulated transcriptional changes in the MKs. This step requires the MKs to enter endomitosis through inhibition of myosin IIB, which is regulated by RUNX1.31 In addition, guanine nucleotide exchange factor-H1 (GEF-H1) and epithelial cell transforming sequence 2 are sequentially downregulated during endomitosis: GEF-H1 during the 2N-4N transition and ECT2 thereafter.32 Subsequent membrane invagination triggers membrane changes through actin assembly via Wiskott-Aldrich syndrome protein (WASP)–WASP-family verprolin-homologous protein signaling.33,34 Simultaneous cytoskeletal GPIbα–filamin A–actin linkage is also crucial for MK maturation, platelet release, and the maintenance of normal platelet morphology.35,36 Finally, migration of MKs from the osteoblastic to the vascular niche (Figure 1) is promoted by the cytokine CXCL12 (formerly known as stromal cell-derived factor 1) and its receptor CXCL receptor 4.37
The best-known morphologic structures occurring during platelet biogenesis are proplatelets.3 Recent studies strongly indicate the involvement of several extracellular matrix proteins in platelet shedding in the perivascular niche, including vWF, fibrinogen, fibronectin, and vascular cell adhesion molecule 1. Of those, Takizawa et al showed the preferential effect of vascular cell adhesion molecule 1 on proplatelets through its interaction with α4β1 integrin on MKs. This effect is particularly apparent when a deficiency of the inhibitory adaptor protein Lnk/Sh2b3, situated downstream of TPO/Mpl signaling in MKs, enhances proplatelet formation.38 Lnk/Sh2b3 deficiency in mice was found to enhance TPO/Mpl signaling, leading to a fivefold to sixfold increase in MK and platelet counts.38 Lnk/Sh2b3 is required for outside-in signaling from platelet integrin αIIbβ3, which contributes to stable thrombus formation in vivo.39 Indeed, in a small number of patients, essential thrombocythemia is caused by Lnk/Sh2b3 dysfunction in combination with JAK2 V617F mutation. In those patients, impairment of this adaptor molecule is accompanied by impaired platelet functionality.40,41 On the other hand, enhanced αIIbβ3 outside-in signaling impairs proplatelet formation in MKs,42 strongly suggesting that diminished outside-in signaling may accelerate proplatelet formation and platelet biogenesis from MKs in mice deficient of or expressing dysfunctional Lnk/Sh2b3.38,43
Cytoskeletal proteins contribute to proplatelet formation by mediating the continuous transport of materials and structural rearrangements necessary to maximize platelet production.44 Once proplatelets enter the bloodstream, blood flow breaks them apart, with each swelling in the tandem line of swellings in a proplatelet containing all the components of a functional platelet.45 Recent advances in in vivo imaging have enabled deeper understanding of platelet shedding from MKs. Two-photon imaging revealed that the directed extension of proplatelets to the sinusoidal vessels is regulated by sphingosine 1-phosphate (S1P) and its receptor, S1pr1. Deficiency in either of these molecules results in proplatelets extending randomly without bias for the bloodstream.46 However, the S1P gradient only attracts proplatelets to the vessels, but does not enable them to breach the endothelial wall and enter the vessels. For that purpose, the WASP-dependent actions of podosomes on the membrane surface are necessary.47
In addition to enhancing our understanding of proplatelets, recent results obtained using in vivo imaging have prompted 2 groups to propose different mechanisms for platelet biogenesis. Kowata et al proposed that immature thick protrusions, not proplatelets, are dominant during the acute phase of thrombocytopenia.48 This proposal attempted to explain recovery from thrombocytopenia through large fragmentations of the membrane. In addition, Nishimura et al independently developed a modified 2-photon imaging system and software to directly observe single platelets within mouse BM, finding an alternative morphologic dynamic, called MK rupture, which rapidly released much larger numbers of platelets.49 Interestingly, this mechanism appears to occur in response to acute needs and to be regulated by elevated interleukin-1α (IL-1α) levels after platelet loss or inflammatory stimulation. Indeed, in a reconstitution study, IL-1α administration rapidly induced MK rupture-dependent thrombopoiesis and increased platelet counts in mice.49 Although there has long been controversy regarding caspase activation or caspase-mediated apoptosis during platelet biogenesis,50 reports finally showed that proplatelet-based platelet biogenesis is independent of the caspase-induced apoptosis pathway in MKs.51,52 However, Nishimura et al revealed that IL-1α/IL-1 type 1 receptor signaling leads to caspase-3 activation, which reduces plasma membrane stability and ultimately leads to MK rupture in a process distinct from typical Fas ligand–induced apoptosis.49 In addition, Kunishima et al detected a heterozygous TUBB1 F260S mutation located at the α- and β-tubulin intradimer interface in a family with congenital macrothrombocytopenia and demonstrated that the resultant disorganized microtubule assembly leads to MK rupture instead of proplatelet formation when the MKs were stimulated with TPO in vitro.53 IL-1α/IL-1 type 1 receptor signaling in MKs may activate a similar mechanism through the disruption of tubulin formation. More interestingly, TPO stimulation of MKs appears to dose-dependently inhibit quick release of platelets by maintaining proplatelets (Figure 1).49 It thus appears that the balance between TPO and IL-1α determines the cellular programming of MKs for controlling platelet number, and if this regulation is impaired, sustained thrombocytopenia may develop.
Genetic defects in the transcription factors regulating MK commitment and differentiation lead to thrombocytopenia
Many thrombocytopenias are now known to arise from genetic abnormalities (Figure 2; supplemental Table 1, available on the Blood Web site). In addition to CAMT, thrombocytopenia with absent radii (TAR) syndrome and radioulnar synostosis with congenital thrombocytopenia are 2 amegakaryocytic thrombocytopenias also characterized by specific skeletal abnormalities. In TAR, microdeletions at 1q21.1 have been detected not only in the majority of patients, but also in their unaffected parents.54 Albers et al recently discovered that the complex inheritance pattern of TAR syndrome is caused by both a low-frequency noncoding single-nucleotide polymorphism and a rare null RBM8A allele, both of which repress transcriptional activity.55 RBM8A encodes the exon-junction complex subunit member Y14. Although the molecular mechanism by which the exon-junction complex regulates both megakaryopoiesis and forelimb development is largely unknown, Fiedler et al revealed defective TPO/Mpl signaling, reflected by JAK2 phosphorylation in pediatric but not in adult MKs.56 In TAR, platelet counts increase with age, spontaneously recovering to near normal levels after the first year of life. Moreover, differences in the characteristics of the fetal and adult MKs suggest differences in their TPO/Mpl signaling properties as well as the platelet biogenesis.18,57 The underlying defect thus appears to be specific for fetal MKs.
Schematic illustration of MK lineage commitment, growth and differentiation, and maturation and platelet release. Causative genes involved in congenital thrombocytopenias are listed as well as in supplemental Table 1. The upper illustration depicts the “genes” in individual developmental stages related to thrombocytopenia in humans. The lower illustration shows the membrane glycoproteins and cytoskeletal structure components within platelets. For example, the physical linkage of the GPIb–filamin A–actin cytoskeleton is responsible for both platelet biogenesis and morphology. Macrothrombocytopenia induced by activating mutations of GPIIb/IIIa (integrin αIIbβ3) is probably caused by myosin IIA–dependent cascade.
Schematic illustration of MK lineage commitment, growth and differentiation, and maturation and platelet release. Causative genes involved in congenital thrombocytopenias are listed as well as in supplemental Table 1. The upper illustration depicts the “genes” in individual developmental stages related to thrombocytopenia in humans. The lower illustration shows the membrane glycoproteins and cytoskeletal structure components within platelets. For example, the physical linkage of the GPIb–filamin A–actin cytoskeleton is responsible for both platelet biogenesis and morphology. Macrothrombocytopenia induced by activating mutations of GPIIb/IIIa (integrin αIIbβ3) is probably caused by myosin IIA–dependent cascade.
Radioulnar synostosis with congenital thrombocytopenia arises due to a truncating mutation in the homeobox gene HOXA11 and is characterized by autosomal-dominant neonatal amegakaryocytic thrombocytopenia and proximal fusion of the radius and ulna.58 Mutant HoxA11 with diminished DNA-binding efficiency impairs MK differentiation in a cell line model,59 whereas deletion of HoxA11 is associated with forelimb abnormalities in a mouse model.60
Moreover, the defects are in the transcription factors that regulate MK lineage commitment, growth, and differentiation. In the context of transcriptional regulation that changes the fate from HSC to MK, transcription factors are critical for the maintenance of proper platelet homeostasis (Figure 2).
Familial platelet disorder with predisposition to acute myeloid leukemia is caused by mutations in RUNX1, the gene encoding the α subunit of the CBF transcription complex.61 CBF is essential for the establishment of definitive hematopoiesis and for regulation of the maintenance, proliferation, and differentiation of HSCs,61 which suggests the expansion of HSCs would predispose one to hematologic malignancies.62 RUNX1 may work not only as a master regulator of hematopoiesis, but also may play a role in lineage decisions at the ME-P stage.27 In addition, RUNX1 may support the switch from mitosis to endomitosis for polyploidization by downregulating the expression of MYH10, the gene encoding nonmuscle myosin heavy chain (NMMHC) IIB (NMMHC-IIB).31 Furthermore, RUNX1 regulates MYH9 and its regulator, MYL9, indicating that defects in familial platelet disorder with predisposition to acute myeloid leukemia are abnormalities of polyploidization, cytoplasmic maturation, and proplatelet formation.31
Point mutations in the 5′ untranslated region of ANKRD26 cause autosomal-dominant thrombocytopenia 2 with predisposition to hematologic malignancies.63 During the late stages of megakaryopoiesis, RUNX1 and FLI1 bind the 5′ untranslated region of ANKRD26, silencing the gene. ANKRD26 mutations result in the loss of RUNX1 and FLI1 binding so that ANKRD26 expression persists, resulting in the constitutive activation of TPO/Mpl signaling, mainly through the mitogen-activated protein kinase pathway.64
ETV6 encodes a transcriptional repressor in the E26 transformation-specific sequence (ETS) family and was initially identified as a tumor suppressor based on its involvement in somatic translocations in childhood leukemia, including ETV6-RUNX1 fusion.65 ETS variant 6 (ETV6) is essential not only for HSC survival but also for MK maturation. Recently, whole-exome sequencing was used to identify heterozygous ETV6 mutations in families with autosomal-dominant thrombocytopenia.66,67 Mutations in the conserved ETS DNA-binding domain showed dominantly reduced transcriptional repression and altered MK maturation.
Jacobsen syndrome and its variant, Paris-Trousseau thrombocytopenia, are congenital disorders caused by partial deletions of 11q23.68,69 The platelet defects include platelet enlargement with large α-granules, which is due to the loss of 1 copy of FLI1 located in the deleted region.70 Point mutations can also cause the disease.71 FLI1 is a member of the ETS family and cooperates with RUNX1 during megakaryopoiesis. In Paris-Trousseau thrombocytopenia, MKs are immature and polyploidization is defective due to aberrant NMMHC-IIB persistence.68,72
GATA1 is a key regulator of erythroid and megakaryocytic homeostasis. Inherited GATA1 mutations cause an X-linked thrombocytopenia with either dyserythropoietic anemia or β-thalassemia.73,74 With GATA1 mutations, MKs are immature and dysplastic, and platelets are large with decreased numbers of α-granules. Mutations in the GATA1/FOG1 interaction domain are associated with dyserythropoietic anemia, whereas those in the DNA-binding surface are associated with β-thalassemia. No FOG1 mutations have yet been reported.
Growth factor independence 1b is a transcriptional repressor containing 6 zinc-finger domains and is differentially expressed during hemato- and lymphopoiesis.75 Two null GFI1B mutations that disrupt DNA binding were identified in patients with autosomal-dominant macrothrombocytopenia, a decrease in α-granules, and red cell anisopoikilocytosis.76,77 The 2 mutants exert dominant-negative effects when coexpressed with the wild-type form. Monteferrario et al77 described the condition as gray platelet syndrome, though the platelets reported by Stevenson et al76 were not gray. Gfi1b mutants dominantly affect the terminal maturation of MKs and subsequent release of platelets with persistent CD34 expression and decreased CD42b expression.77 In addition to GFI1B, mutations causing a reduction in α-granules have also been reported in ANKRD26 and GATA1, and a complete loss of α-granules is caused by VPS33B78 and NBEAL2 mutations,79-81 indicating the involvement of multiple molecular pathways in α-granule biogenesis.
Genetic defects affecting the maturation of MKs and platelet release
Platelet size is a feature typically used when diagnosing a congenital thrombocytopenia disorder. In most of these ailments, the platelets are large, though platelet size is reduced in Wiskott-Aldrich syndrome and X-linked thrombocytopenia caused by WAS mutations.82 MK maturation and proplatelet formation require dynamic reorganization of the actomyosin cytoskeleton and microtubules. Consequently, defects in platelet cytoskeletal organization and/or related signaling pathways are associated with abnormal proplatelet formation.83,84 The platelet membrane glycoproteins and platelet cytoskeletal networks mutated in congenital macrothrombocytopenias are illustrated in Figure 2.
Platelets are released via proplatelet formation from MKs and subsequently mature and even divide when in circulation.45,85 Key components of platelet release processes are microtubules assembled from α- and β-tubulin heterodimers. Among the β-tubulin isoforms, β1-tubulin is MK/platelet-specific and the predominant isoform.86 TUBB1 mutations interfere with proper microtubule assembly and result in proplatelet-independent platelet release, that is, MK rupture. Lacking marginal bands, platelets from patients with TUBB1-related macrothrombocytopenia are large and spherical.53,87
MYH9 disorder/MYH9-related disease is an autosomal-dominant disorder characterized by macrothrombocytopenia, leukocyte inclusion bodies, and a risk of developing progressive nephritis, deafness, and cataracts. Mutations in MYH9, the gene encoding NMMHC-IIA, cause this disease.88,89 Although May-Hegglin anomaly, the prototype MYH9 disorder, was originally thought to be very rare, the advent of specific immunofluorescence analysis has enabled the detection of abnormal NMMHC-IIA aggregates within the cytoplasm of granulocytes and more precise diagnosis and classification90 ; thus, MYH9 disorders are now known to be the most prevalent congenital thrombocytopenia. NMMHC-IIA consists of an N-terminal globular head domain that binds to actin and hydrolyses adenosine triphosphate, a coiled coil rod domain and a C-terminal nonhelical tailpiece. NMMHC-IIA (myosin IIA) dimerizes at the rod domain to form bipolar functional filaments, and 2 pairs of myosin light chains bind to its head domain.91 A dominant-negative effect of abnormal NMMHC-IIA expression is the molecular basis for inclusion bodies within granulocytes composed of ribonucleoprotein complexes consisting of MYH9 messenger RNA, NMMHC-IIA polypeptides, and ribosomes.92,93 The mechanisms underlying the production of large platelets are somewhat paradoxical. MK differentiation and maturation appear to be normal; only proplatelet formation is affected.94,95 MKs from patients and knock-in mouse models show decreased numbers of proplatelet tips, increases in proplatelet size, and shortened and enlarged proplatelet shafts.95-97 Myosin IIA negatively regulates proplatelet formation through Rho/Rho-associated protein kinase signaling mediated by the interaction between integrin α2β1 and type I collagen, which is enriched in the osteoblastic niche.98,99 Accordingly, myosin IIA prevents premature platelet release from MKs that have not reached the vascular niche, and defects in functional myosin IIA frees immature MKs to undergo ectopic platelet release. In addition, Spinler et al recently showed that pre/proplatelet fission is lacking in MYH9 disorder platelets.100 Collectively, these findings indicate that macrothrombocytopenia in MYH9 disorders must be the result of premature platelet release and cytofission failure.
ACTN1 is a recently identified gene that encodes α-actinin 1, and mutations within the gene were confirmed to be a frequent cause of autosomal-dominant macrothrombocytopenia.101,102 α-Actinin 1 dimers stabilize the actin filaments and contribute to actin cytoskeletal organization by crosslinking actin filaments. ACTN1 mutations are localized mostly within the functional actin-binding and calmodulin-like domains. Furthermore, they cause disorganization of actin-based cytoskeletal structures, defective proplatelet branching, and the production of abnormally large but fewer proplatelet tips101 (Figure 2).
Similarly, dysregulation of myosin IIA arises from integrin αIIbβ3 (GPIIb/IIIa) gene mutations. It is well known that homozygous mutations in ITGA2B or ITGB3 result in a bleeding disorder, Glanzmann thrombasthenia, in which both homozygous patients and heterozygous carriers have normal platelet counts and morphology.103 Recently, heterozygous activating mutations in the membrane-proximal region of the αIIb and β3 subunit were found to be associated with congenital macrothrombocytopenia.42,104,105 These mutations disrupt the electrostatic interaction between the cytoplasmic tails of αIIb and β3, resulting in a constitutively active receptor and downstream focal adhesion kinase phosphorylation.42 Focal adhesion kinase is known to inhibit Rho and may promote precocious proplatelet formation, which is common among MYH9 disorders.98,99 However, some ITGA2B- and ITGB3-activating mutations are not associated with macrothrombocytopenia, indicating different molecular mechanisms for the induction of abnormal proplatelet formation.105
In addition, congenital deficiency in GPIb/IX/V, the platelet vWF receptor, results in Bernard-Soulier syndrome, a rare bleeding disorder that is accompanied by macrothrombocytopenia.106 Loss of GPIb/IX/V from the platelet membrane disables platelets from adhering to the walls of injured vessels, thus patients suffer from bleeding problems. The cytoplasmic domain of GPIbα associates with actin via filamin A. Because the density of GPIb/IX affects platelet size, it has been proposed that defective physical linkage between GPIb/IX/V and the cytoskeleton is responsible for large platelets.107,108 In addition, recent studies indicate that proplatelet formation is defective in Bernard-Soulier syndrome MKs, though the cells do not exhibit maturation defects,109,110 suggesting that defective interactions between GPIb/IX and filamin A-actin adversely affect both platelet morphogenesis and megakaryocytopoiesis. This same effect is true for filamin A deficiency in patients with an FLNA mutation or in FLNA-knockout mice.111,112 In this context, Manchev et al found a homozygous missense mutation in PRKACG (p.I74M), which encodes the γ-catalytic subunit of cAMP-dependent protein kinase.36 Because PRKACG is responsible for filamin A phosphorylation at Ser2152, which protects filamin A from proteolysis, the mutation results in filamin A degradation and disruption of proplatelet formation.36 Macrothrombocytopenia is also seen in platelet-type von Willebrand disease (VWD) and type 2B VWD, which, respectively, arise from dominant gain-of-function mutations in GP1BA and VWF.113,114 Furthermore, proplatelet formation is inhibited in type 2B VWD MKs, and this effect is mimicked by the binding of anti-GPIbα antibodies to MKs.115,116 Finally, vWF binding to GPIb/IX induces signal transduction and inside-out signaling to integrin αIIbβ3 (GPIIb/IIIa).117 These findings reinforce the notion that not only a physical interaction between GPIb/IX and the cytoskeleton but also vWF-GPIb/IX-mediated signaling affects megakaryocytopoiesis and platelet biogenesis (Figure 2).
Conclusion and perspective
New insight into normal megakaryopoiesis has deepened our understanding of congenital thrombocytopenia and vice versa. Furthermore, the recent advent of next-generation sequencing technologies and microscopy are together helping to identify novel mechanisms involved in megakaryopoiesis and thrombopoiesis. We anticipate that the identification of unknown factors and the mechanisms by which these factors act under physiological and pathophysiological conditions will continue to bring us closer to a full understanding of megakaryopoiesis.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by a Grant-in-aid Kiban type from the Ministry of Education, Culture, Sports, Science and Technology (K.E. and S.K., respectively), the Project of Realization of Regenerative Medicine and Highway from the Japan Agency for Medical Research and Development (K.E.), and the Initiative for Accelerating Regulatory Science in Innovative Drug, Medical Device, and Regenerative Medicine from the Ministry of Health, Labor and Welfare (K.E.).
Authorship
Contribution: K.E. designed the review construct and wrote updated mechanisms of normal platelet biogenesis; and S.K. wrote updated genetic disorders inducing thrombocytopenia.
Conflict-of-interest disclosure: K.E. has a financial interest and is a founder of Megakaryon Co Ltd, a company that aims to produce donor-independent human platelets from human-induced pluripotent stem cells. The interests of K.E. were reviewed and were approved by Kyoto University in accordance with their conflict-of-interest policies. S.K. declares no competing financial interests.
Correspondence: Koji Eto, Department of Clinical Application Research, Center for iPS Cell Research and Application, Kyoto University, Kyoto 606-8507, Japan; e-mail: kojieto@cira.kyoto-u.ac.jp.