Key Points
ITP patients differ in their tendency to bleed despite similarly low platelet counts, thereby confounding treatment decisions.
Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP patients.
Abstract
Immune thrombocytopenia (ITP) patients with similarly low platelet counts differ in their tendency to bleed. To determine if differences in platelet function in ITP patients account for this variation in bleeding tendency, we conducted a single-center, cross-sectional study of pediatric patients with ITP. Bleeding severity (assessed by standardized bleeding score) and platelet function (assessed by whole blood flow cytometry) with and without agonist stimulation was evaluated in 57 ITP patients (median age, 9.9 years). After adjustment for platelet count, higher levels of thrombin receptor activating peptide (TRAP)-stimulated percent P-selectin- and activated glycoprotein (GP)IIb-IIIa–positive platelets were significantly associated with a lower bleeding score, whereas higher levels of immature platelet fraction (IPF), TRAP-stimulated platelet surface CD42b, unstimulated platelet surface P-selectin, and platelet forward light scatter (FSC) were associated with a higher bleeding score. Thus, platelet function tests related to platelet age (IPF, FSC) and activation through the protease activated receptor 1 (PAR1) thrombin receptor (TRAP-stimulated P-selectin, activated GPIIb-IIIa, and CD42b), independent of platelet count, are associated with concurrent bleeding severity in ITP. These tests may be useful markers of future bleeding risk in ITP.
Introduction
Approximately 5000 new cases of pediatric immune thrombocytopenia (ITP) are diagnosed each year in the United States,1 with ∼80% of cases resolving spontaneously within 3 to 6 months, with the remaining 20% persisting for more than 6 to 12 months. Most patients, even those with severe thrombocytopenia (platelet counts <20 × 109/L), present with only mild bruising and petechiae, but up to 20% of children suffer more significant bleeding with extensive mucosal bleeding, and 0.4% experience intracranial hemorrhage.2 The American Society of Hematology and international consensus guidelines on the management of primary ITP state that most children with newly diagnosed ITP may be managed with close observation without specific therapy at the discretion of the treating clinician and family.3,4 Pharmacologic treatment of pediatric ITP is directed toward rapidly increasing the platelet count with first-line treatments (eg, corticosteroids, IV immunoglobulin [Ig], or anti-D), or other treatments including immunosuppressive agents (eg, rituximab, mycophenolate, and 6-mercaptopurine) or thrombopoietin receptor agonists.4,5 The decision of whether or not to treat ITP with pharmacologic agents must account for the variable efficacy of treatment, treatment-related toxicities, health-related quality-of-life issues related to preventing and monitoring bleeding, and the risk, albeit low, of life-threatening bleeding complications.
A major dilemma for the treating physician is that ITP patients differ in their tendency to bleed despite similarly low platelet counts. However, there are scant data addressing the question of whether tests of platelet function can predict bleeding in patients with ITP,6,7 because most tests of platelet function are affected by thrombocytopenia. However, platelet function measured by whole blood flow cytometry is largely unaffected by the platelet count because individual platelets are analyzed one at a time.8 In the present study, we therefore sought to determine whether whole blood flow cytometric tests of platelet function, independent of platelet count, are associated with bleeding severity in children with ITP.
Methods
Study design and patient selection
The relationship between bleeding scores and platelet function tests was investigated in a single-center, cross-sectional study of pediatric patients with ITP. The study was approved by the Boston Children’s Hospital Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Male and female patients over 6 months of age, in the pediatric hematology clinic or admitted to the hospital with a diagnosis of ITP or Evans syndrome were eligible for enrollment. All eligible patients, without regard to bleeding severity, with a scheduled clinical blood draw between the hours of 8 am and 5 pm on weekdays from March 2012 through January 2014, and from November 2014 through April 2015, were invited to participate.
Bleeding scores, platelet count, mean platelet volume (MPV), and immature platelet fraction (IPF)
Following informed consent, blood was collected into EDTA and 3.2% sodium citrate vacuum tubes, and current bleeding was graded using the Buchanan and Adix bleeding score.9 Bleeding was scored by one of three hematologists blinded to platelet function results, and investigators performing platelet function tests were blinded to clinical results. To avoid factors that might confound study results, the study blood draw was not performed around other procedures (eg, IV placements or bone marrow aspirates) for any of the patients. EDTA-anticoagulated blood was used for measurement of platelet counts and MPV in an Advia automated complete blood count analyzer (Siemens USA, Deerfield, IL) and for measurement of IPF by a Sysmex XE-2100 hematology analyzer (Sysmex Corporation, Kobe, Japan). Samples were maintained at room temperature between collection and IPF analysis, which was performed within 1 hour of sample collection.
Flow cytometric analysis of platelet markers
Whole blood flow cytometric analysis of platelet surface P-selectin and activated glycoprotein (GP)IIb-IIIa was performed essentially as previously described10,11 using sodium citrate-anticoagulated blood. Briefly, aliquots of whole blood (10 µL) were incubated (15 minutes at room temperature) with fluorescently labeled monoclonal antibodies (10 µL) and either 0.5 µM adenosine 5′-diphosphate (ADP) (Bio/Data, Horsham, PA), 20 µM ADP, 1.5 µM protease activated receptor 1 (PAR1) thrombin receptor-activating peptide (TRAP) SFLLRN-amide (Bachem, Torrance, CA), 20 µM TRAP, or N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-Tyrode’s buffer (10 mM HEPES, 137 mM sodium chloride, 2.8 mM potassium chloride, 1 mM magnesium chloride, 12 mM sodium hydrogen carbonate, 0.4 mM sodium phosphate dibasic, 5.5 mM glucose, and 0.35% w/v bovine serum albumin, pH 7.4) (5 µL). The reaction was stopped with a 15-fold dilution in 1% formaldehyde in HEPES-saline buffer. The antibodies used were: phycoerythrin (PE)–conjugated anti–P-selectin monoclonal antibody (1.5 µg/mL final concentration, CD62P, clone AK4; BD Pharmingen); fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody PAC1 (40 µg/mL final concentration; BD Biosciences), which only binds to the activated conformation of GPIIb/IIIa12 ; and PE-Cy5–conjugated anti-CD42b (GPIb) monoclonal antibody (40 µg/mL final concentration, clone HIP1; BD Pharmingen). PE-conjugated MIgG1 isotype (1.5 µg/mL final concentration; BD Pharmingen) and FITC-PAC1 together with 2.5 µg/mL of the GPIIb/IIIa antagonist eptifibatide to block specific binding, served as the negative controls for platelet surface P-selectin and platelet surface activated GPIIb/IIIa, respectively.
Procoagulant platelet-derived microparticles
Platelet-derived microparticles with surface exposed phosphatidylserine were measured as previously described.13 Briefly, sodium citrate anticoagulated whole blood was diluted with an equal volume buffer with Gly-Pro-Arg-Pro (25 mM final concentration; Sigma-Aldrich) and 20 µL of the mixture added to 20 µL of either (a) calcium chloride 10 mM in 10 mM HEPES-Tyrode’s buffer, or the same buffer with (b) convulxin 200 ng/mL (Centerchem Inc, Norwalk, CT), (c) equine tendon type I collagen 12.5 µg/mL (Chrono-Log, Havertown, PA) and epinephrine 5 µM (Bio/Data, Horsham, PA), or (d) ADP 20 µM and TRAP 20 µM. After incubation (15 minutes at 37°C), the sample was mixed with 20 µL of a cocktail of Annexin V–FITC (1:60 final dilution), PE–CD41a (1:15 final dilution), and PE-Cy5–CD42b (1:10 final dilution, all from BD Pharmingen) and incubated a further 15 minutes at room temperature, then fixed by the addition of 800 µL 1% formaldehyde. A negative control for nonspecific Annexin V–FITC binding was prepared for each subject by omitting calcium from the reaction mixture and this was used to set the marker for the percent positive region. Flow cytometric analysis was performed in a Becton Dickinson FACSCalibur as previously described.13 The results are reported as % platelet-derived microparticles: the percentage of all CD41a and CD42b double-positive events with low forward light scatter (FSC) (relative to unstimulated platelets) that also bind Annexin V.
Statistical analysis
Data were analyzed using SAS software, version 9.2 or 9.3 (SAS Institute, Cary, NC) and GraphPad Prism version 5.0a (GraphPad Software, La Jolla, CA). Normally distributed data (as judged by the D’Agostino and Pearson omnibus normality test) are summarized as mean ± standard deviation (SD). Nonparametric data are reported as median and interquartile range (IQR). For statistical analysis and graphical representation, patients with bleeding scores of 3 or 4 have been grouped. The univariate association of each platelet function test with bleeding score (4 categories: bleeding score of 3 or 4, 2, 1, and 0) was assessed by ordinal logistic regression in SAS. Bonferroni correction was used to account for multiple comparisons with P values ≤.0017 (.05/30 tests) considered significant. Tests that were significant at the univariate level were evaluated in a model, which included platelet count as a covariate (adjusted). For this model, Bonferroni correction was again used to account for multiple comparisons with P values ≤.01 (.05/5 tests) considered significant.
Results
Patient characteristics
Patient characteristics are shown in Table 1. Fifty-seven patients (31 male, age 9.9 ± 5.4 years [mean ± SD], median platelet count 59, IQR 14-125 × 109/L) were enrolled. All patients included in the study met the standard criteria for a diagnosis of ITP,4 including a platelet count of <100. However, treatment of some patients at the time of the blood draw resulted in platelet counts above the threshold used in the original diagnosis. One subject was hospitalized and the remaining 56 subjects were outpatients. ITP was newly diagnosed (<3 months) in 20 patients, persistent (3 to 12 months) in 9 patients, and chronic (>12 months) in 28 patients. Steroids were the most common treatment in the 30 days immediately prior to enrollment in the study. Buchanan and Adix bleeding score (higher number indicates worse bleeding) on the day of enrollment was “0” in 18 patients, “1” in 22 patients, “2” in 11 patients, “3” in 5 patients, and “4” in 1 patient. Patient characteristics associated by univariate analysis with a high bleeding score on the day of blood draw included female gender (P = .018, odds ratio [OR] 3.4 [1.2-9.1]) and ITP duration (P = .0024 overall, P = .0055 for newly diagnosed vs chronic ITP, OR 8.3 [2.5-27.2]). Age, treatment during the 30 days prior to blood draw, and antecedent viral illness were not significantly associated with bleeding score by univariate analysis (P > .05 for each).
Patient characteristics and associations with bleeding score
| . | . | Univariate association with bleeding score . | Association with bleeding score adjusted for platelet count . | ||
|---|---|---|---|---|---|
| Parameter . | N, Mean or Median . | P . | OR per unit or 1 SD (95% CI) . | P . | OR per unit or 1 SD (95% CI) . |
| Gender | Female, n = 26 | .0180 | Female vs male; 3.35 (1.23-9.13) | .1137 | — |
| Male, n = 31 | |||||
| Age (y) | 9.9 ± 5.4 | .5260 | — | — | — |
| Ethnicity | Not Hispanic, n = 39 | .4107 | — | — | — |
| Hispanic, n = 7 | |||||
| Unknown, n = 11 | |||||
| Platelet count (× 109/L) | 59 (IQR 14-125)* | .0080 | 0.925† (0.874-0.980) | — | — |
| ITP duration | New (<3 mo), n = 20 | .0024 | New vs chronic; 8.26 (2.51-27.2) | .0002 | New vs chronic; 16.2 (4.29-61.0) |
| Persistent (3-12 mo), n = 9 | |||||
| Chronic (>12 mo), n = 28 | |||||
| Diagnosis | Primary (n = 47), Evans (n = 7), Non-Evans Secondary (n = 3) | .0222 | Primary vs Evans; 18.9 (2.03-175) | — | — |
| Medical treatment | Any (n = 25) vs | .5933 | — | — | — |
| None (n = 32) | |||||
| Steroids (n = 15) vs | .7923 | — | — | — | |
| Not steroids, n = 42 | |||||
| Antecedent viral illness | No, n = 29 vs | .0919 | — | — | — |
| Yes, n = 18 | |||||
| Unknown, n = 10 | |||||
| . | . | Univariate association with bleeding score . | Association with bleeding score adjusted for platelet count . | ||
|---|---|---|---|---|---|
| Parameter . | N, Mean or Median . | P . | OR per unit or 1 SD (95% CI) . | P . | OR per unit or 1 SD (95% CI) . |
| Gender | Female, n = 26 | .0180 | Female vs male; 3.35 (1.23-9.13) | .1137 | — |
| Male, n = 31 | |||||
| Age (y) | 9.9 ± 5.4 | .5260 | — | — | — |
| Ethnicity | Not Hispanic, n = 39 | .4107 | — | — | — |
| Hispanic, n = 7 | |||||
| Unknown, n = 11 | |||||
| Platelet count (× 109/L) | 59 (IQR 14-125)* | .0080 | 0.925† (0.874-0.980) | — | — |
| ITP duration | New (<3 mo), n = 20 | .0024 | New vs chronic; 8.26 (2.51-27.2) | .0002 | New vs chronic; 16.2 (4.29-61.0) |
| Persistent (3-12 mo), n = 9 | |||||
| Chronic (>12 mo), n = 28 | |||||
| Diagnosis | Primary (n = 47), Evans (n = 7), Non-Evans Secondary (n = 3) | .0222 | Primary vs Evans; 18.9 (2.03-175) | — | — |
| Medical treatment | Any (n = 25) vs | .5933 | — | — | — |
| None (n = 32) | |||||
| Steroids (n = 15) vs | .7923 | — | — | — | |
| Not steroids, n = 42 | |||||
| Antecedent viral illness | No, n = 29 vs | .0919 | — | — | — |
| Yes, n = 18 | |||||
| Unknown, n = 10 | |||||
P values are for Wald χ square; P < .05 was considered significant.
Platelet count is given as median and IQR.
OR and 95% CI are for each increase in platelet count of 10 × 109/L.
To determine whether patient characteristics identified as being associated with bleeding score by univariate analysis were associated with bleeding severity independent of platelet count, ordinal logistic regression analyses were performed including platelet count as a fixed factor. Following adjustment for platelet count, gender was no longer significantly associated with bleeding score, whereas duration of ITP, specifically newly diagnosed vs chronic ITP, remained significantly associated with bleeding score (P = .0002, OR 16.2 [4.3-61.0]) (Table 1).
Association of IPF, MPV, and FSC with the bleeding score
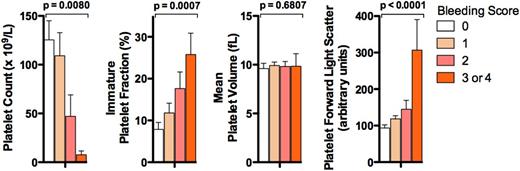
Figure 1 shows the platelet count, IPF, MPV, and platelet FSC levels stratified by bleeding score. As expected, higher platelet counts were significantly associated by univariate analysis with lower bleeding scores (P = .0080, OR 0.93 per 10 × 109/L increase in platelet count [95% CI, 0.87-0.98]) (Figure 1), whereas higher IPF levels were associated with higher bleeding scores (P = .0007, OR 2.5/1 SD [11.6 units] increase (95% CI, 1.47-4.3]). MPV was not significantly associated with bleeding score (P = .68; Figure 1). In contrast, high platelet FSC, which increases with particle size and therefore is influenced by MPV, was significantly associated with higher bleeding scores (P < .0001, OR 6.8/1 SD [99.0 units] increase [95% CI, 2.7-17.6]) (Figure 1).
Association of platelet laboratory parameters with bleeding score. Results shown are the means ± SEM for the platelet count, IPF, MPV, and platelet FSC in ITP patients stratified by bleeding score (bleeding score 0, n = 18; 1, n = 22; 2, n = 11; and 3 or 4, n = 6). P values (above each data set) are for univariate ordinal logistic regressions with respect to bleeding score.
Association of platelet laboratory parameters with bleeding score. Results shown are the means ± SEM for the platelet count, IPF, MPV, and platelet FSC in ITP patients stratified by bleeding score (bleeding score 0, n = 18; 1, n = 22; 2, n = 11; and 3 or 4, n = 6). P values (above each data set) are for univariate ordinal logistic regressions with respect to bleeding score.
Following adjustment for platelet count, IPF remained significantly associated with bleeding scores (P = .0296, OR 2.1 [95% CI, 1.14.0]), as did platelet FSC (P = .0012, OR 5.5 [95% CI, 2.0-15.4]).
Response of ITP patient platelets to in vitro agonist stimulation
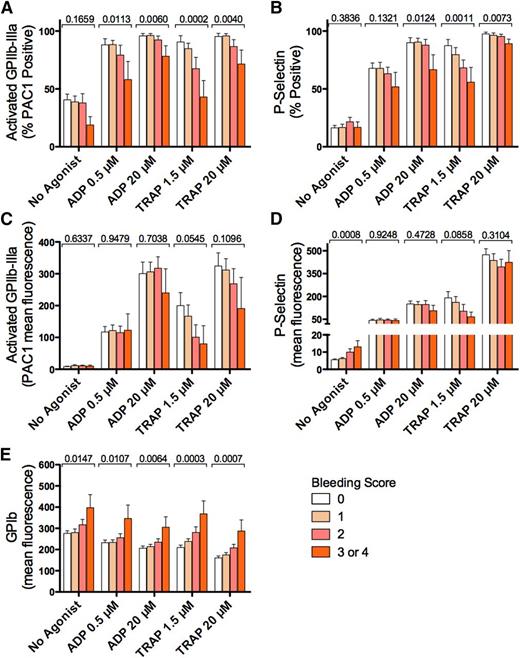
As expected,8 platelet surface activated GPIIb-IIIa and platelet surface P-selectin in whole blood from ITP patients stimulated with ADP (0.5 µM or 20 µM) or the platelet PAR1 agonist, TRAP (1.5 µM or 20 µM) were significantly increased compared with unstimulated samples (Table 2; P < .05 for agonist-stimulated compared with “No Agonist”; Dunn’s multiple comparison test). Similarly, as expected,8,14-16 platelet surface GPIb was significantly reduced by in vitro stimulation with ADP or TRAP compared with unstimulated platelets in blood (Table 2; P < .05 vs “No Agonist”).
Platelet surface marker response of ITP patient platelets to in vitro agonist stimulation
| . | No agonist . | ADP 0.5 µM . | ADP 20 µM . | TRAP 1.5 µM . | TRAP 20 µM . |
|---|---|---|---|---|---|
| Activated GPIIb-IIIa, % PAC1- positive | 38.5 (15.8, 52.2) | 93.2 (86.5, 96.9) | 97.8 (95.7, 98.5) | 95.2 (65.3, 97.3) | 97.8 (95.1, 98.8) |
| P-selectin, % positive | 14.7 (8.8, 25.8) | 67.4 (57.0, 80.7) | 93.1 (87.4, 97.4) | 88.4 (61.6, 97.4) | 98.8 (96.9, 99.3) |
| Activated GPIIb-IIIa, PAC1 MFI | 8.1 (5.1, 11.7) | 103.6 (54.8, 182.9) | 275.8 (189.8, 414.6) | 103.5 (30.5, 214.1) | 269.2 (154.5, 422.6) |
| P-selectin, MFI | 5.8 (4.9, 7.7) | 36.6 (22.5, 51.3) | 114.6 (82.0, 207.6) | 79.5 (32.6, 210.3) | 417.3 (335.9, 504.0) |
| GPIb, MFI | 298.4 (244.1, 346.3) | 227.8 (205.5, 282.8) | 220.7 (190.3, 248.3) | 239.0 (192.0, 303.5) | 171.9 (149.8, 225.5) |
| . | No agonist . | ADP 0.5 µM . | ADP 20 µM . | TRAP 1.5 µM . | TRAP 20 µM . |
|---|---|---|---|---|---|
| Activated GPIIb-IIIa, % PAC1- positive | 38.5 (15.8, 52.2) | 93.2 (86.5, 96.9) | 97.8 (95.7, 98.5) | 95.2 (65.3, 97.3) | 97.8 (95.1, 98.8) |
| P-selectin, % positive | 14.7 (8.8, 25.8) | 67.4 (57.0, 80.7) | 93.1 (87.4, 97.4) | 88.4 (61.6, 97.4) | 98.8 (96.9, 99.3) |
| Activated GPIIb-IIIa, PAC1 MFI | 8.1 (5.1, 11.7) | 103.6 (54.8, 182.9) | 275.8 (189.8, 414.6) | 103.5 (30.5, 214.1) | 269.2 (154.5, 422.6) |
| P-selectin, MFI | 5.8 (4.9, 7.7) | 36.6 (22.5, 51.3) | 114.6 (82.0, 207.6) | 79.5 (32.6, 210.3) | 417.3 (335.9, 504.0) |
| GPIb, MFI | 298.4 (244.1, 346.3) | 227.8 (205.5, 282.8) | 220.7 (190.3, 248.3) | 239.0 (192.0, 303.5) | 171.9 (149.8, 225.5) |
Results shown are medians (IQR), n = 41. Each platelet marker was significantly altered by in vitro stimulation (P < .0001, 2-sided nonparametric analysis of variance; P < .05 for each agonist compared with “No Agonist”; Dunn’s multiple comparison test).
MFI, geometric mean fluorescence intensity.
Association of platelet surface activated GPIIb-IIIa, P-selectin, and GPIb with the bleeding score
Figure 2 shows the levels of platelet surface activated GPIIb-IIIa, P-selectin, and GPIb stratified by bleeding score and the P values (univariate analysis) for association of each marker with the bleeding score. ITP patient blood assessed following in vitro stimulation with ADP or TRAP, at low or high concentration, showed higher percentages of platelets positive for activated GPIIb-IIIa in patients with lower bleeding scores (univariate P < .05 for all) (Figure 2A). Similarly, the percentages of P-selectin–positive platelets following in vitro stimulation with 20 µM ADP or 1.5 µM or 20 µM TRAP were higher in patients with lower bleeding scores (univariate P < .05 for all) (Figure 2B). In each case, higher levels of platelets positive for activated GPIIb-IIIa or P-selectin were associated with lower bleeding scores. In contrast, the amount of activated GPIIb-IIIa exposed on the surface of each platelet, as judged by the geometric MFI of bound activation-dependent monoclonal antibody PAC1, was not significantly associated with bleeding score with or without in vitro stimulation (Figure 2C). However, on circulating (no agonist) platelets, relatively higher levels of platelet surface P-selectin (Figure 2D) were associated with higher bleeding scores (P = .0008) (Figure 2D). Platelet surface GPIb with and without in vitro stimulation was higher in patients with higher bleeding scores (Figure 2E; univariate P < .05 for all). The associations of the bleeding score with TRAP 1.5 µM stimulated percent activated GPIIb-IIIa–positive (P = .0002)(Figure 2A), percent P-selectin–positive platelets (P = .0011) (Figure 2B), platelet surface CD42b MFI with 1.5 µM TRAP (P = .0032), and 20 µM TRAP (P = .0035) with bleeding score were significant at the Bonferroni corrected P value of ≤ .0017.
Platelet activation markers, with and without in vitro stimulation, stratified by bleeding score in ITP patients. (A-B) Percentages of unstimulated (No Agonist), ADP-, and TRAP-stimulated activated GPIIb-IIIa–positive and P-selectin–positive platelets, respectively, in ITP patient blood, stratified by bleeding score (bleeding score 0, n = 18; 1, n = 22; 2, n = 11; and 3 or 4, n = 6). (C-E) Platelet surface density (as measured by geometric MFI) of activated GPIIb-IIIa (C), P-selectin (D), and GPIb (E) on unstimulated and in vitro-stimulated platelets. Results shown are means ± SEM. P values (above each data set) are for univariate ordinal logistic regressions with respect to bleeding score.
Platelet activation markers, with and without in vitro stimulation, stratified by bleeding score in ITP patients. (A-B) Percentages of unstimulated (No Agonist), ADP-, and TRAP-stimulated activated GPIIb-IIIa–positive and P-selectin–positive platelets, respectively, in ITP patient blood, stratified by bleeding score (bleeding score 0, n = 18; 1, n = 22; 2, n = 11; and 3 or 4, n = 6). (C-E) Platelet surface density (as measured by geometric MFI) of activated GPIIb-IIIa (C), P-selectin (D), and GPIb (E) on unstimulated and in vitro-stimulated platelets. Results shown are means ± SEM. P values (above each data set) are for univariate ordinal logistic regressions with respect to bleeding score.
Association of procoagulant platelet-derived microparticles with the bleeding score
The percent platelet-derived microparticles was significantly increased following in vitro stimulation with collagen/epinephrine or ADP/TRAP (P < .05 Dunn’s multiple comparison test; see supplemental Table 1 on the Blood Web site) but not after stimulation with convulxin. However, Annexin-positive platelet-derived microparticles were not significantly associated with the bleeding score either with or without in vitro stimulation (univariate P > .05 for all) (supplemental Figure 1).
Association of platelet function tests with bleeding severity independent of platelet count
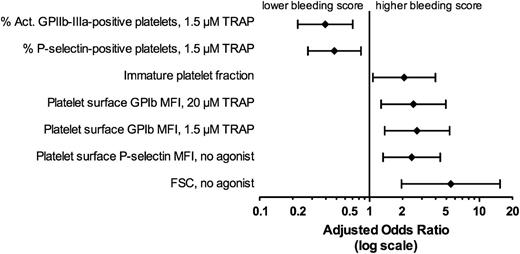
As described above, the platelet function tests that were found to be significantly associated with higher bleeding scores by univariate analysis at the Bonferroni corrected P value of ≤ .0017 (to correct for multiple comparisons) were: IPF (P = .0007), platelet FSC (P < .0001), no agonist platelet surface P-selectin MFI (.0008), percent activated GPIIb-IIIa–positive, TRAP 1.5 µM (P = .0002), percent P-selectin–positive platelets, TRAP 1.5 µM (P = .0011), platelet surface CD42b MFI with 1.5 µM TRAP (P = .0003), and 20 µM TRAP (P = .0007) (Figures 1 and 2). Figure 3 shows the association of the explanatory variables identified as significant in univariate analysis with bleeding score after adjustment for the platelet count. Following adjustment for platelet count, each of these end points remained significantly associated with bleeding score (P values, ORs, and 95% CI for 1 SD change in each end point are provided in supplemental Table 2). Likewise, each of these end points remained significantly associated with bleeding score (P < .05 for all, data not shown) after adjustment for additional possible confounding factors: diagnosis (primary, Evans, non-Evans secondary), medical treatment (none, any), antecedent viral illness, gender, age, and ethnicity.
Association of explanatory variables identified as significant in univariate analysis with bleeding score after adjustment for the platelet count. ORs and 95% CIs per 1 SD unit (defined below) increase in the indicated parameter. One SD unit for each test is as follows: Act. GPIIb-IIIa–positive platelets, 1.5 µM TRAP, 29.2%; P-selectin–positive platelets, 1.5 µM TRAP, 27.6%; IPF, 11.6%; platelet surface CD42b MFI, 20 µM TRAP, 65.8 geometric mean fluorescence units; platelet surface GPIb MFI, 1.5 µM TRAP, 82.9 geometric mean fluorescence units; platelet surface P-selectin MFI, no agonist, 4.9 geometric mean fluorescence units; FSC, no agonist, 99.0 FSC units.
Association of explanatory variables identified as significant in univariate analysis with bleeding score after adjustment for the platelet count. ORs and 95% CIs per 1 SD unit (defined below) increase in the indicated parameter. One SD unit for each test is as follows: Act. GPIIb-IIIa–positive platelets, 1.5 µM TRAP, 29.2%; P-selectin–positive platelets, 1.5 µM TRAP, 27.6%; IPF, 11.6%; platelet surface CD42b MFI, 20 µM TRAP, 65.8 geometric mean fluorescence units; platelet surface GPIb MFI, 1.5 µM TRAP, 82.9 geometric mean fluorescence units; platelet surface P-selectin MFI, no agonist, 4.9 geometric mean fluorescence units; FSC, no agonist, 99.0 FSC units.
Similarly, the same platelet function tests were also found to be significantly associated by univariate analyses with bleeding scores when only results from patients with platelet counts <100 × 109/L at the time of sampling were analyzed (bleeding scores, n = 39: 0, n = 9; 1, n = 14; 2, n = 10; and 3 or 4, n = 6): IPF (P = .0345), platelet FSC (P = .0028), platelet surface P-selectin MFI, no agonist (P = .0036), PAC1 % positive, 1.5 µM TRAP (P = .0014), P-selectin % positive, 1.5 µM TRAP (P = .0103), and platelet surface CD42b MFI with 1.5 µM TRAP (P = .0032) and 20 µM TRAP (P = .0035).
Discussion
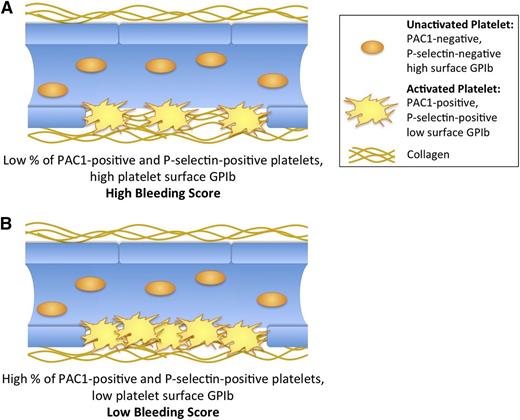
In this single-site, cross-sectional study of pediatric ITP patients, we identified platelet biomarkers, which independent of platelet count are associated with bleeding severity on the day of the blood draw. Specifically, higher levels of TRAP 1.5 µM-stimulated percent P-selectin–positive and activated GPIIb-IIIa–positive platelets were significantly associated with lower bleeding scores (Figure 3; supplemental Table 2), whereas higher levels of TRAP 1.5 and 20 µM-stimulated platelet surface CD42b MFI were significantly associated with higher bleeding scores (Figure 3; supplemental Table 2). Because the normal response to TRAP stimulation of the platelet PAR1 thrombin receptor is an increase in platelet surface P-selectin and activated GPIIb-IIIa and a decrease in platelet surface CD42b, the association of all of these tests with bleeding suggests a mechanistic link between reduced thrombin receptor-mediated activation of platelets and bleeding events (Figure 4). In ITP patients with a greater predisposition to bleeding, activation of a lower percentage of platelets at sites of vascular injury may result in incomplete coverage of exposed subendothelium (Figure 4A) and/or reduced thrombus stability, despite a similar number of circulating platelets compared with ITP patients with a lesser predisposition to bleeding (Figure 4B).
Cartoon representation of platelet activation markers associated with bleeding score independent of platelet count in ITP patients. (A) Patients with high bleeding scores, where low concentration TRAP yields a low percentage of PAC1- and P-selectin–positive platelets. (B) Patients with low bleeding scores, where low concentration TRAP yields a high percentage of PAC1- and P-selectin–positive platelets. (A-B) thus represent patients with different bleeding tendencies despite an equal number of circulating platelets.
Cartoon representation of platelet activation markers associated with bleeding score independent of platelet count in ITP patients. (A) Patients with high bleeding scores, where low concentration TRAP yields a low percentage of PAC1- and P-selectin–positive platelets. (B) Patients with low bleeding scores, where low concentration TRAP yields a high percentage of PAC1- and P-selectin–positive platelets. (A-B) thus represent patients with different bleeding tendencies despite an equal number of circulating platelets.
Although the phase of ITP was significantly associated with bleeding score, platelet function tests were not significantly different between patients with different ITP duration. Thus, the platelet tests associated with bleeding score appear to be applicable to patients of differing ITP duration.
Serious bleeding, especially intracranial bleeding, is rare in pediatric ITP.17,18 International and American Society of Hematology guidelines therefore suggest no pharmacologic treatment in the absence of bleeding in pediatric ITP.3,4 Despite these guidelines, treatment-related toxicities, variable efficacy of treatment, and treatment costs, treatment is frequently initiated in the absence of bleeding due to physician and family concerns regarding the potentially serious nature of these infrequent events. The presently described markers of ITP bleeding risk could potentially inform the decision of whether or not to institute pharmacologic treatment in these patients.
Reduced ability to respond to TRAP suggests a possible defect in platelet signaling at or downstream from PAR1. TRAP stimulates release of ADP, which contributes to the overall response to TRAP through activation of platelet ADP receptors.19 Thus, defects in platelet response to ADP could also contribute to the reduced response in TRAP-stimulated platelet function markers in ITP patients with high bleeding scores. Although not significant at the Bonferroni corrected P value of .0017, the low univariate P values for association of ADP-stimulated percent P-selectin–positive and activated GPIIb-IIIa–positive platelets with bleeding scores (Figure 2) suggest that the signaling defect in ITP patients may not be limited to the PAR1 pathway.
IPF, a marker of young platelets,20 was significantly associated with the bleeding score by univariate analysis and after adjustment for platelet count. Immature platelets are often considered to be larger than older platelets,21 and IPF is defined in part on the basis of high FSC,22 which is strongly influenced by particle size. However, determination of platelet size is confounded by differences in methodologies, and some studies report a significant negative correlation between IPF and MPV.23 In the present study, MPV showed a significant positive correlation with IPF (P = .02). However, MPV was not significantly associated with the bleeding score by univariate analysis (Figure 1). In contrast, platelet FSC, which partly reflects platelet size but also depends on refractive index, granularity, and shape of the particle,8,24 was significantly associated with bleeding score, even after adjustment for platelet count (Figure 3; supplemental Table 2). Whether the individual factors that contribute to increased platelet FSC, such as increased platelet refractive index (eg, due to changes in membrane lipid composition), decreased platelet granularity (eg, due to release of platelet granule contents), or altered platelet shape (eg, due to pseudopodia extension) are independently associated with bleeding severity in ITP and requires further investigation.
In the present study, higher levels of platelet surface P-selectin on unstimulated platelets were associated with higher bleeding scores (Figure 3). This finding may reflect elevated P-selectin on the surface of circulating platelets in vivo or pre-analytical activation induced by sample handling before flow cytometry25 despite efforts to minimize this effect. Increased surface expression of P-selectin detected by whole blood flow cytometry on platelets of ITP patients was reported as early as 199126 and correlated with the amount of platelet-associated IgG.26 Protein A immunoadsorption therapy reduced platelet associated IgG and IgM in ITP patients and also reduced the percentage of circulating P-selectin–positive platelets, suggesting an immune mechanism for the increased circulating P-selectin–positive platelets.27 Panzer et al reported an increased percentage of circulating P-selectin–positive platelets in adult chronic ITP patients28 and that ITP patients with high platelet surface P-selectin in vivo showed reduced agonist-induced platelet activation.29 Panzer et al subsequently reported6 that P-selectin expression on circulating platelets of ITP patients did not correlate with bleeding score, whereas platelet count and von Willebrand factor-dependent platelet adhesion were inversely associated with bleeding score. In contrast, we report that increased levels of P-selectin on the surface of unstimulated platelets and reduced TRAP-induced platelet activation in ITP patients are significantly associated with higher bleeding scores even after adjustment for platelet counts (Figure 3; supplemental Table 2). A likely explanation for the difference in findings is that we used whole blood flow cytometry to study platelet activation whereas Panzer et al6,29 performed flow cytometric analysis on platelet-rich plasma, the preparation of which is prone to introduce artifactual platelet activation.8
In a recent study,7 van Bladel et al reported that pediatric chronic ITP patients with a severe bleeding phenotype demonstrated decreased platelet co-aggregation with normal donor platelets in response to phorbol myristate acetate, compared with patients with a mild bleeding phenotype. The aggregation response of normal donor platelets by the novel platelet aggregation method used by van Bladel et al ranged widely, raising concerns that the procedures associated with their method may introduce variation. Nevertheless, the concept of reduced platelet aggregation in response to activation in patients with a more severe bleeding phenotype is consistent with our results of reduced TRAP-induced percent platelets with activated GPIIb-IIIa in patients with high bleeding scores (Figure 3; supplemental Table 2). Similar to the nonsignificant trends we observe here (Figure 2), van Bladel et al7 reported decreased ADP-induced platelet surface P-selectin measured by flow cytometry in patients with higher bleeding scores.
Like previous studies of pediatric ITP,6,7,29 limitations of the present study include small sample size, few subjects with high bleeding scores, and variation in pharmacologic treatment. However, our finding of platelet tests associated with bleeding scores independent of platelet count in consecutively enrolled ITP patients regardless of treatment suggests that a larger study with more patients with higher bleeding scores and more uniform treatment would yield an even more robust result. The presence of antiplatelet antibodies was not evaluated in the present study because such antibodies are not sensitive or specific for the diagnosis of pediatric ITP.3-5 Because GPIIb-IIIa is the most common target of antiplatelet antibodies found in ITP5 and reduced percent platelets positive for activated GPIIb-IIIa in the presence of 1.5 µM TRAP was associated with higher bleeding scores in this study, we considered the possibility that PAC1 binding was blocked by autoantibodies in patients with high bleeding scores. However, autoantibodies directed at platelet receptors cannot account for the present results, because the same patients with reduced binding of PAC1 also had reduced binding of fluorescent antibodies directed against platelet surface P-selectin (Figures 2B and 3) and increased binding of fluorescent antibodies directed against GPIb (Figures 2E and 3). We also considered the possibility that the reduced levels of P-selectin- and activated GPIIb-IIIa–positive platelets following TRAP or ADP stimulation in patients with high bleeding scores was the result of desensitization associated with the recent bleeding event. However, platelet activation results in a reduction in platelet surface GPIb levels through both internalization and cleavage from the platelet surface,14-16 yet GPIb levels were elevated on circulating platelets of patients with high bleeding scores (Figure 2E). These results raise the possibility that decreased platelet reactivity, which accounts for all the findings, is an intrinsic property of these ITP patients’ platelets. Candidate molecules to account for this reduced reactivity include all those that participate in platelet signaling and activation downstream of both the thrombin (PAR1) and ADP platelet receptors. Whether this apparent decreased reactivity is consistent over time and is associated with the severity of subsequent bleeding events in ITP patients remains to be determined. Follow-up studies will therefore focus on testing the consistency of the identified platelet biomarkers over time and whether they are predictive of future bleeding severity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the statistical support of Carter Petty, Harvard Catalyst Biostatistics Program.
Authorship
Contribution: A.L.F. and R.F.G. conceived the studies, analyzed and interpreted the data, and wrote the manuscript; A.J.G., M.A.B.-L., T.B., and S.L.C. performed the research and contributed to the manuscript; and E.J.N. and A.D.M. conceived the studies, interpreted the data, and provided critical revisions to the manuscript.
Conflict-of-interest disclosure: A.D.M. and A.L.F. are principal investigators on a research grant to Boston Children’s Hospital from Sysmex. The remaining authors declare no competing financial interests.
Correspondence: Andrew L. Frelinger III, Center for Platelet Research Studies, Division of Hematology/Oncology, Boston Children’s Hospital, Karp 07212, 300 Longwood Ave, Boston, MA 02115; e-mail: andrew.frelinger@childrens.harvard.edu.
References
Author notes
A.L.F. and R.F.G. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal