Key Points
Triple therapy is well tolerated and effective in patients with chronic ITP.
Relapse free survival was 92% for responders after 12 months and 76% after 24 months.
Abstract
Promising reports of combination immunosuppression with high-dose dexamethasone and rituximab for the treatment of primary immune thrombocytopenia (ITP) have recently emerged. They suggest a potential to further optimize the efficacy of therapy. We investigate the use of a novel combination of conventional therapies in ITP given over 4 weeks. From 2011 to 2014, 20 patients were prospectively enrolled onto a single-arm phase 2b study to describe the safety, efficacy, and tolerability of oral dexamethasone 40 mg for days 1 to 4, oral cyclosporine 2.5 to 3 mg/kg daily for day 1 to 28, and intravenous low-dose rituximab 100 mg for days 7, 14, 21, and 28. There were no therapy-related serious adverse side effects, 6-month response rate was 60%, and treatment was well tolerated. Responders enjoyed relapse-free survivals of 92% and 76%, respectively, at 12 and 24 months. This study highlights the possibility of achieving an enduring remission from 4 weeks of therapy. This study is registered at www.anzctr.org.au (#ANZCTRN12611000015943).
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by isolated thrombocytopenia in the absence of other causes.1 ITP is mediated by antiplatelet autoantibodies. Antibody-coated platelets are phagocytosed by macrophages in the reticuloendothelial system, leading to accelerated platelet clearance.2 Macrophages also act as antigen-presenting cells interacting with CD8+ and CD4+ T cells that in turn stimulate antibody-producing B cells.3 This pathogenic loop sustains autoantibody production. T cell-mediated platelet lysis4 and megakaryocyte immunoinjury contribute to the diverse pathobiology of ITP.5
Single-agent treatments have not been successful at inducing prolonged remission.6 With immunosuppressive monotherapy, ITP patients usually require prolonged treatment, leading to unpleasant and sometimes serious side effects.7,8
Recent studies combining dexamethasone and rituximab in short courses have reported encouraging results.9-13 We postulate that adding cyclosporine to this combination may induce a more enduring remission by also targeting T cells and thereby briefly suppressing all 3 immune cell types implicated in sustaining the pathogenic loop.
Suppressing these cells simultaneously has a risk of predisposing to serious infections. We considered it appropriate to conduct a pilot study on a small number of patients with the aim of investigating the safety and efficacy of the triple therapy.
Study design
Twenty patients were randomly but nonconsecutively and prospectively enrolled onto a phase 2b study investigating triple therapy: oral dexamethasone 40 mg for days 1 to 4, oral cyclosporine 2.5 to 3 mg/kg daily for days 1 to 28, and intravenous low-dose rituximab 100 mg for days 7, 14, 21, and 28 (TT4). There was no loading dose for cyclosporine, trough levels were monitored weekly for toxicity, and doses were titrated to target 200 to 400 μg/L. Additional cycles of dexamethasone were permitted if response was delayed. This study protocol was approved by the South Eastern Sydney Local Health District Human Research Ethics Committee and conducted in accordance with the Declaration of Helsinki.
Eligibility and end points
ITP patients ≥18 years of age were eligible to participate. The primary objective was to investigate the safety, and to a lesser degree, the efficacy of TT4. The primary hematologic end point was 6-month response rate (RR). We used the criteria of the International Working Group and the American Society of Hematology practice guideline panel for ITP diagnosis and response.1,14,15 Treatment-free survival (TFS) is defined as the time from TT4 protocol to the introduction of further therapy for symptomatic or severe (<20×109/L) thrombocytopenia.16 The supplemental Data available on the Blood Web site provides the inclusion and exclusion criteria, as well as secondary end points. Adverse side effects were monitored regularly by an independent safety review committee and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events scale, version 4.02.17
Statistical methods
This phase 2b study was designed to terminate if sequential monitoring of therapy-related serious adverse events revealed a higher than expected frequency using a concave α-spending function.18 Subgroups were compared using Fisher’s exact test for categorical data and the Mann-Whitney U test for quantitative data. Exploratory logistic regressions were applied to 6-month RR for cyclosporine levels, quantitative lymphocyte changes, and baseline demographics including weight and body surface area (BSA) by Mostellar. Wilcoxon matched-pairs signed-rank tests were used for changes in lymphocytes counts. Kaplan-Meier survival curves were compared using Mantel-Cox log-rank testing for response durability, TFS, and time to CD19+ lymphocyte recovery. Tests were 2-sided, and P < .05 was considered statistically significant.
Results and discussion
Safety
Demographics are presented in Table 1. There were no deaths, therapy-related serious adverse events, serum sickness, treatment interruptions, or delays caused by toxicity. There were 4 therapy-related grade III to IV adverse side effects: 3 patients with hypertension and 1 patient with wound infection. These side effects were treated successfully. Concurrent use of high-dose steroids and cyclosporine may have contributed to the hypertension observed. Other toxicities are described in supplemental Data.
Baseline characteristics and initial platelet response to therapy
| Parameter . | Responders . | Nonresponders . | P . |
|---|---|---|---|
| Demographics | |||
| Median age (range) | 50.2 (18.9-79.8) | 51.9 (19.3-67.5) | .9394 |
| Males/females | 10/2 | 1/7 | .0045 |
| Median time from diagnosis to TT4, months | 60 (1-206) | 42 (3-215) | .8345 |
| Newly diagnosed or persistent ITP | 3 | 4 | .3563 |
| Chronic ITP | 9 | 4 | |
| Primary ITP | 9 | 6 | 1.0000 |
| Secondary ITP | SLE | SLE | |
| APS | APS | ||
| Crohns disease | |||
| 2 or less prior lines of therapy | 8 | 0 | .0047 |
| 3 or more prior lines of therapy | 4 | 8 | |
| Median BSA | 1.98 (1.65-2.39) | 1.70 (1.48-2.29) | .1878 |
| Median weight, kg | 80.(61-114) | 67 (48-105) | .2779 |
| Splenectomy, prior | 1 | 1 | |
| Platelets | |||
| Baseline, median ×109/L | 18.5 (4-35) | 16.0 (4-24) | .2114 |
| Day 7, median | 109.0 (1-322) | 45.0 (9-114) | .0861 |
| Day 14, median | 91.5 (6-413) | 25 (9-252) | .0566 |
| Day 21, median | 92.5 (14-393) | 27.5 (8-79) | .0051 |
| Day 28, median | 144.5 (22-337) | 18.5 (9-131) | .0021 |
| Day 60, median | 108 (23-316) | 20 (1-61) | .0008 |
| Patients with platelets >30 × 109/L, day 30 | 11 | 3 | .0181 |
| Patients with platelets >30 × 109/L, at day 60 | 11 | 1 | .0017 |
| Parameter . | Responders . | Nonresponders . | P . |
|---|---|---|---|
| Demographics | |||
| Median age (range) | 50.2 (18.9-79.8) | 51.9 (19.3-67.5) | .9394 |
| Males/females | 10/2 | 1/7 | .0045 |
| Median time from diagnosis to TT4, months | 60 (1-206) | 42 (3-215) | .8345 |
| Newly diagnosed or persistent ITP | 3 | 4 | .3563 |
| Chronic ITP | 9 | 4 | |
| Primary ITP | 9 | 6 | 1.0000 |
| Secondary ITP | SLE | SLE | |
| APS | APS | ||
| Crohns disease | |||
| 2 or less prior lines of therapy | 8 | 0 | .0047 |
| 3 or more prior lines of therapy | 4 | 8 | |
| Median BSA | 1.98 (1.65-2.39) | 1.70 (1.48-2.29) | .1878 |
| Median weight, kg | 80.(61-114) | 67 (48-105) | .2779 |
| Splenectomy, prior | 1 | 1 | |
| Platelets | |||
| Baseline, median ×109/L | 18.5 (4-35) | 16.0 (4-24) | .2114 |
| Day 7, median | 109.0 (1-322) | 45.0 (9-114) | .0861 |
| Day 14, median | 91.5 (6-413) | 25 (9-252) | .0566 |
| Day 21, median | 92.5 (14-393) | 27.5 (8-79) | .0051 |
| Day 28, median | 144.5 (22-337) | 18.5 (9-131) | .0021 |
| Day 60, median | 108 (23-316) | 20 (1-61) | .0008 |
| Patients with platelets >30 × 109/L, day 30 | 11 | 3 | .0181 |
| Patients with platelets >30 × 109/L, at day 60 | 11 | 1 | .0017 |
There was 1 hospitalization for influenza: an 80-year-old man living alone. One patient required intravenous antibiotics for a lower limb laceration. One patient was admitted with reversible renal failure from nonsteroidal anti-inflammatory drugs 18 months after TT4 treatment. One nonresponding patient remained steroid dependent after failing further therapies and presented with pulmonary aspergilloma 23 months after TT4.
Efficacy
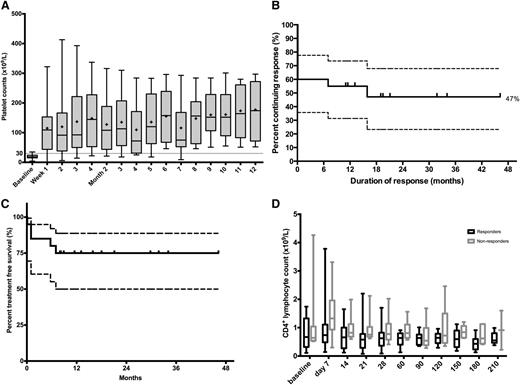
Overall, 12 of 20 patients (60%) responded. All maintained their response for ≥7 months. Median time to response was 7.4 days. Complete response was 30% at 6 months. Only 2 responders relapsed during a median follow-up of 17.5 months (range, 7-47 months). For responders, relapse-free survival at 12 and 24 months was 92% and 76%, respectively (95% confidence interval [CI], 53-98% and 30-93%, respectively). See Figure 1 for details.
Platelet response, Kaplan Meier analyses and CD4+ lymphocyte depletion. (A) Whisker plots of platelet counts during the first 12 months from responders. The central horizontal bold line is the median; the cross is the mean; the lower and upper box limits are the first and third quartiles, respectively; and the whiskers include all data points. One patient relapsed at month 7. (B) Kaplan-Meier analysis of all patients treated with TT4. Vertical marks indicate the last follow-up of an ongoing response; 47% of the 20 treated patients were estimated to have a long-term response of >46 months from treatment. (C) Kaplan-Meier TFS curve for all patients. Median undefined, 75% treatment free at 12 months (95% CI, 49-88%). (D) Whisker plots of CD4+ T cells during the first 7 months: responders vs nonresponders. Median 0.62 vs 0.91 (P < .0001). The central horizontal line is the median; the lower and upper box limits are the first and third quartiles, respectively; and the whiskers include all data points. CD4+ counts were not significantly lower in men compared with women (P = .1639).
Platelet response, Kaplan Meier analyses and CD4+ lymphocyte depletion. (A) Whisker plots of platelet counts during the first 12 months from responders. The central horizontal bold line is the median; the cross is the mean; the lower and upper box limits are the first and third quartiles, respectively; and the whiskers include all data points. One patient relapsed at month 7. (B) Kaplan-Meier analysis of all patients treated with TT4. Vertical marks indicate the last follow-up of an ongoing response; 47% of the 20 treated patients were estimated to have a long-term response of >46 months from treatment. (C) Kaplan-Meier TFS curve for all patients. Median undefined, 75% treatment free at 12 months (95% CI, 49-88%). (D) Whisker plots of CD4+ T cells during the first 7 months: responders vs nonresponders. Median 0.62 vs 0.91 (P < .0001). The central horizontal line is the median; the lower and upper box limits are the first and third quartiles, respectively; and the whiskers include all data points. CD4+ counts were not significantly lower in men compared with women (P = .1639).
Two nonresponders underwent splenectomy: 1 patient at 5 months achieved complete response and another patient at 3 years after completion of TT4 therapy achieved reduction in steroid dependence. Despite failing the response criteria, further therapy was not required for 4 of 8 nonresponders. Overall, TFS was 75% at 12 months (95% CI, 49-88%).
Two responders received an additional dexamethasone cycle: one within the first 30 days because of a delayed response and another within the first 6 months when platelets transiently fell with a coryzal illness. The other 10 of 12 responders did not receive additional cycles of dexamethasone. Six of 8 nonresponders received an additional cycle of high-dose dexamethasone.
The following subgroups were associated with 6-month RR: male sex (P = .0045), <3 prior lines of therapy (P = .0047), and an initial platelet response by day 28 (P = .0181) or day 60 (P = .0017). There was no association between response and BSA, despite the use of fixed low-dose rituximab.
Odds ratios for male patients to be responders were 35.0 (95% CI, 2.6-465; P = .007). Cyclosporine levels, BSA, lymphocyte depletion, immunoglobulin levels, prior therapies, and other baseline demographics did not significantly influence the patients’ response.
T cells
From day 7 to day 28, peripheral CD4+ T cells fell by a median of 0.41 × 109/L (95% CI, 0.05-0.63 × 109/L; P = .0032) for all patients, irrespective of response. However, responders had lower CD4+ T cells than nonresponders for 6 months after treatment (median, 0.62 vs 0.91 × 109/L; P < .0001).
B cells
Peripheral CD19+ B cells became undetectable for all patients by day 28. B-cell recovery was earlier for patients <50 years (median, 6.5 months vs not reached; P = .0105), but no such associations were found with response status, weight, or BSA. Median time to recovery was 14.9 months. Duration and depth of B-cell depletion did not influence patient response (P = .8999 and P = .8911, respectively). These results suggest a therapeutic ceiling effect from peripheral B-cell depletion not seen with CD4+ T-cell suppression.
Immunoglobulins
Two patients had preexisting hypogammaglobulinemia: immunoglobulin (Ig)G levels fell from 6.6 to 2.1 g/L for 1 patient, but they remained between 5.0 and 6.0 g/L for the other (reference range, 7.0-16.0 g/L). Two patients developed transient hypogammaglobulinemia. Overall, IgG levels fell from baseline (median, 0.97 g/L for 6 months after treatment; 95% CI, 0.09-3.2 g/L; P = .0093). IgM levels fell (median, 0.20 g/L; 95% CI, 0.06-0.45 g/L; P = .0068). No changes were observed in IgA levels. No correlation was observed between immunoglobulin levels and response.
Discussion
Interpretation of our study is limited by the sample size. However, we did not observe excessive toxicity. TT4 was well tolerated by all patients including 4 patients >65 years of age. Most grades I to II therapy-related adverse side effects were attributable to high-dose dexamethasone and resolved by day 7.
Our study included 12 of 20 patients (60%) who failed ≥3 prior lines of therapy. Steroid resistance was notably increased in 4 of the 7 patients who had 2 prior lines of therapy. These cases are all less likely to respond or spontaneously remit than treatment-naïve ITP.9,11
Although treatment with TT4 was more successful for patients who had failed <3 lines of therapy, long-standing disease was not a disadvantage. The improved outcome for men in our study has not been reported in studies previously using rituximab either alone19 or in combination with dexamethasone.10 Our study suggests that male ITP patients may benefit from T-cell suppression, but this requires confirmation by further studies.
As CD4+ T cell activation is part of the pathogenic loop sustaining ITP, suppressing their activation seems a reasonable target. Supporting this hypothesis, cyclosporine monotherapy has demonstrated efficacy in chronic and refractory ITP.20,21 In these studies, platelet counts increased within 4 weeks of commencement. In our study, CD4+ counts fell during this time. Although dexamethasone alone may cause some T cell suppression, we postulate a synergism with cyclosporine that may lead to improved platelet responses, at least for a subset of ITP patients.
We included 5 patients with secondary ITP. Treatment of their underlying conditions was inadequate to control their thrombocytopenia. Excluding their data had no significant impact on the study outcomes.
Despite fixed low-dose rituximab, we were surprised to see no disadvantage with increasing BSA or male sex. The latency of response reported with low-dose rituximab22 may be ameliorated by adding dexamethasone.11
A major advantage of TT4 is its short duration of therapy (28 days) and yet 60% of patients enjoyed prolonged remissions of ≥7 months without further therapy. Protocols using low-dose rituximab will be attractive where funding resources are limited. Although our study shows encouraging results, the incremental benefit of cyclosporine to rituximab and dexamethasone remains unresolved, and randomized controlled trials are required.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Roslyn Ristuccia for assistance in study protocol design and Elena Simon Mendoza for assistance in coordinating study and collecting data; both are from the St George Clinical Trial Unit. The authors also thank Dr Amanda Hugman, Professor Szu-Hee Lee, Dr Freda Passam, and Dr Stephanie Hayes for assistance in conducting the study.
Authorship
Contribution: P.Y.-I.C. designed the study protocol, coordinated the study, collected, analyzed, and interpreted the data, wrote the first draft of the manuscript, and edited the manuscript; F.R. provided intellectual and clinical input and edited the manuscript; X.B. provided intellectual and clinical input and edited the manuscript; S.R. provided intellectual and clinical input and edited the manuscript; S.-J.H. provided intellectual input and edited the manuscript; and B.H.C. conceived the idea, designed the study protocol, analyzed and interpreted the data, and edited the manuscript.
Conflict-of-interest disclosure: X.B. has received speaker’s fees from Roche. B.H.C. is on the speakers bureau and receives research funding from GSK and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Beng H. Chong, Level 4, Clinical Services Building, St George Hospital, Gray St, Kogarah, NSW 2217, Australia; e-mail: beng.chong@unsw.edu.au.