Key Points
Dysregulated IL-1α in NADPH oxidase null (Cybb KO) mice initiated increased G-CSF–induced neutrophilia, exacerbating sterile inflammation.
Reduction of early neutrophilic response promoted resolution in Cybb KO mice.
Abstract
The leukocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase generates reactive oxygen species essential in microbial killing and regulation of inflammation. Inactivating mutations in this enzyme lead to chronic granulomatous disease (CGD), associated with increased susceptibility to both pyogenic infections and to inflammatory disorders. The role of the NADPH oxidase in regulating inflammation driven by nonmicrobial stimuli is poorly understood. Here, we show that NADPH oxidase deficiency enhances the early local release of interleukin-1α (IL-1α) in response to damaged cells, promoting an excessive granulocyte colony-stimulating factor (G-CSF)–regulated neutrophilic response and prolonged inflammation. In peritoneal inflammation elicited by tissue injury, X-linked Cybb-null (X-CGD) mice exhibited increased release of IL-1α and IL-1 receptor –mediated G-CSF production. In turn, higher levels of systemic G-CSF increased peripheral neutrophilia, which amplified neutrophilic peritoneal inflammation in X-CGD mice. Dampening early neutrophil recruitment by neutralization of IL-1α, G-CSF, or neutrophil depletion itself promoted resolution of otherwise prolonged inflammation in X-CGD. IL-1β played little role. Thus, we identified an excessive IL-1α/G-CSF response as a major driver of enhanced sterile inflammation in CGD in the response to damaged cells. More broadly, these results provide new insights into the regulation of sterile inflammation, and identify the NADPH oxidase in regulating the amplitude of the early neutrophilic response.
Introduction
The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a multisubunit enzyme highly expressed in granulocytes, monocytes, and macrophages, where its rapid assembly and activation on plasma or phagosome membranes generates superoxide. Inactivating mutations in the NADPH oxidase result in chronic granulomatous disease (CGD), an immunodeficiency associated with recurrent bacterial and fungal infections concomitant with loss of NADPH oxidase–derived superoxide and downstream reactive oxygen species (ROS).1 X-linked recessive mutations in CYBB (gp91phox or NOX2), a membrane flavocytochrome–mediating electron transfer, account for two-thirds of CGD (X-linked Cybb-null [X-CGD]), whereas autosomal-recessive CGD results from inactivating mutations in any of the 4 other enzyme subunits. Apart from their function in microbial killing, NADPH oxidase–derived ROS play complex roles in immune regulation. A distinctive component of CGD is its association with inflammatory conditions, often unrelated to infection, including granulomatous inflammation in the gastrointestinal and genitourinary tracts, discoid lupus-like lesions, and macrophage activation syndrome.2-6 Moreover, NADPH oxidase gene variants are linked to lupus, rheumatoid arthritis, and inflammatory bowel disease.7-10 However, insights into the role of NADPH oxidase in regulating inflammatory responses to sterile endogenous ligands are lacking, an important question given the association of NADPH oxidase deficiency with noninfectious inflammatory disorders.
“Sterile inflammation” is an important component of many autoimmune diseases, trauma, ischemic or toxic injury, and is primarily driven by endogenous danger-associated molecular patterns (DAMPs). DAMPS, including purine metabolites and nucleic acids, are released from damaged cells and are sensed by host innate immune receptors to initiate production of proinflammatory mediators and recruitment of inflammatory leukocytes.11,12 The swift deployment of neutrophils to inflamed sites, within hours, is driven by local and systemic events. Granulocyte colony-stimulating factor (G-CSF) and CXC chemokine receptor 2 (CXCR2) chemokine ligands produced within inflamed tissue act systemically to induce neutrophil mobilization, the rapid upregulation of neutrophil release from the marrow storage pool, to increase neutrophil numbers in the bloodstream.13,14 Circulating neutrophils then extravasate into inflamed tissue in response to local chemoattractant gradients. However, excessive neutrophil recruitment can be detrimental, resulting in tissue damage and/or prolonged inflammation.15
The proinflammatory cytokines interleukins 1α and 1β (IL-1α and IL-1β) play a central role in sterile inflammation.16,17 Although IL-1α and IL-1β both bind and signal through the ubiquitously expressed IL-1 receptor (IL-1R) to activate production of downstream chemokines and cytokines, they can play nonredundant roles in inflammatory diseases due to their differential expression and regulation.17 IL-1β is expressed primarily on myeloid cells, is induced by agonists including microbial ligands or DAMPs, and is processed from its inactive precursor by inflammasome-activated caspase-1 or by other proteases for activity.18 In contrast, IL-1α is widely expressed and can be released from preformed stores following cell damage,19,20 although recent studies have highlighted a more complex regulation for its expression and secretion by myeloid cells in response to DAMPs while still maintaining their integrity.19,21 In particular, the early release of IL-1α from sentinel peritoneal macrophages in response to injected necrotic cells or uric acid crystals drives neutrophil recruitment by inducing the production of inflammatory mediators from surrounding nonhematopoietic cells.19,22-24
In the current study, we show that DAMP-induced peritoneal injury in X-CGD mice resulted in excessive local production of IL-1α, leading to increased G-CSF levels. Consequently, X-CGD mice had increased peripheral neutrophilia and excessive neutrophilic exudation into the peritoneum leading to prolonged inflammation. Dampening this early neutrophilia using neutralizing antibodies for IL-1α or G-CSF, or by depleting neutrophils, promoted subsequent resolution of inflammation. IL-1β played little role. Thus, we identified an excessive IL-1α/G-CSF–driven neutrophil response in NADPH oxidase deficiency as a central initial event that amplifies and prolongs DAMP-induced inflammation.
Methods
Mice
C57Bl/6J and B6.SJL-PtrcaPep3b/BoyJ mice were purchased from The Jackson Laboratory. Mice with inactivation of X-linked Cybb (X-CGD mice)25 in C57Bl/6J (backcrossed >15 generations) or B6.SJL-PtrcaPep3b/BoyJ26 backgrounds were obtained from in-house colonies. C57Bl/6J IL-1R knockouts (IL-1R−/−) were provided by Robyn Klein (Department of Medicine, Washington University School of Medicine, St. Louis, MO). Gata6flox/flox mice on a mixed 129S1/SvImJ and CD-1 background were bred with Lyz2-Cre+/− on a C57BL/6 background to yield Cre+/− Gata6ΔMac mice and Cre−/− Gata6flox/flox littermate control mice as described.27 Mice were maintained in specific pathogen-free conditions and used between 8 and 14 weeks of age. All experiments were conducted as approved by the Washington University in St. Louis Animal Studies Committee.
Peritonitis
Sterile peritonitis was induced by intraperitoneal (i.p.) injection of either 1 mL of 5 mM sodium periodate,28 3 mg of monosodium urate (MSU) crystals, or 20 × 106 necrotic EL-4 lymphocytes prepared as described.22 Postinjection, blood for analysis of blood counts and serum was obtained and peritoneal cells harvested by lavage with phosphate-buffered saline (PBS) with 2 mM EDTA. For cytokine array analysis, mice were lavaged with a single injection of 3 mL of PBS-EDTA. For neutralization experiments, mice were injected IV with either 0.5 mg of anti-IL-1α, 0.5 mg of anti-IL-1β or isotype control antibodies (BioXcell), or 100 μg of anti-G-CSF antibody (Peprotech) as previously described,22,29 1 hour before induction of peritonitis. Neutrophils were depleted by a single IV dose of 0.5 mg of anti-Ly6G (BioXcell) antibody 36 hours before peritonitis.30
In vitro IL-1 assays
To determine responses to necrotic cell ligands, resident macrophages were challenged with necrotic EL-4 cell lysates for 18 hours as described.22 For other agonists, resident macrophages, bone marrow–derived macrophages (BMDMs), and bone marrow–derived dendritic cells (BMDCs) were primed with 10 ng/mL ultrapure lipopolysaccharide (LPS) for 3 hours followed by stimulation with 2 mM adenosine triphosphate (ATP) for 30 minutes or with 50 μg/mL MSU crystals or Silica for 4 hours. Cell-free supernatants were collected after challenge and IL-1α and IL-1β levels determined by enzyme-linked immunosorbent assay (ELISA).
Flow cytometry of peritoneal cells
Antibodies were from BD Pharmingen unless noted. Peritoneal lavage cells were labeled with anti-mouse fluorochrome-conjugated Siglec F, B220, CD3, Ly6C, Ly6G, F4/80, CD115, B220, major histocompatibility complex (MHC) class II, CD206 (Santa Cruz Biotechnology) antibodies. NADPH oxidase assays included oxidation of dihydrorhodamine 123 (DHR).31 Data were collected on FACSCanto (BD Biosciences) or Cytek-modified FACScan (BD Biosciences and Cytek Development) instruments and analyzed with FlowJo (Tree Star). Peritoneal lavage cells were also analyzed by cytospins and Wright-Giemsa staining. Resident peritoneal macrophages were sorted based on the ImmGen consortium gating strategy32 as F4/80hiCD115+B220−MHCII− cells using a high-speed MoFlow cell sorter.
Statistics
Statistical analyses used GraphPad Prism 6.0 (GraphPad Software Inc). A P < .05 was considered statistically significant. A detailed description of statistical tests used is stated in each figure legend.
Additional details are in supplemental Methods (available on the Blood Web site).
Results
NADPH oxidase deficiency resulted in elevated neutrophil and IL-1α response to sterile peritoneal injury
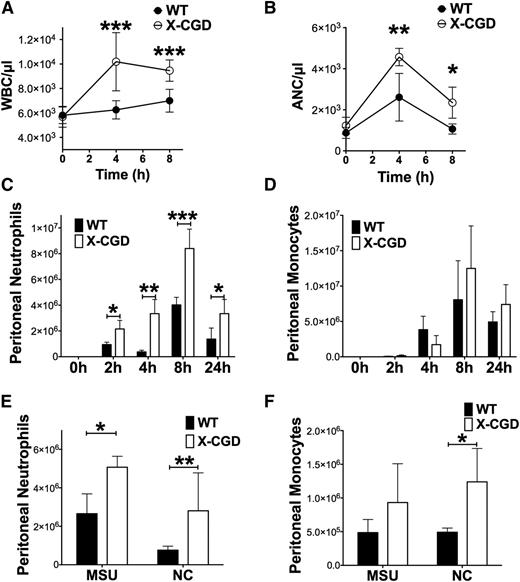
Following i.p. instillation of sodium periodate, a mild oxidizing agent, we previously demonstrated that inflammation in X-CGD mice is exaggerated and prolonged.28 In the acute phase of inflammation following the induction of peritonitis, X-CGD mice exhibited significantly greater peripheral leukocytosis at 4 hours and 8 hours, associated with ≈twofold higher absolute neutrophil counts (ANCs) compared with wild-type (WT) mice (Figure 1A-B). Neutrophilia was accompanied by rapid accumulation of neutrophils (Ly6G+Ly6Cint cells) (Figure 1C; supplemental Figure 1) in the peritoneal cavity, which was significantly higher in X-CGD mice as early as 2 hours and remained higher than WT mice even at 24 hours (Figure 1C-D). However, there was not a significant difference in monocyte accumulation between genotypes in the first 24 hours of inflammation (Figure 1D). We also injected mice with necrotic cell lysates22 or MSU crystals, a canonical DAMP. In either model of exogenous DAMP-induced inflammation, X-CGD mice had significantly elevated peritoneal neutrophil accumulation compared with WT mice (Figure 1E), and monocyte accumulation in response to necrotic cells was also significantly increased (Figure 1F). Taken together, these results show that NADPH oxidase deficiency enhances the acute inflammatory response to tissue injury and exogenous DAMPs, reflected primarily as an exaggerated neutrophil response.
X-CGD mice exhibited enhanced response to sterile tissue injury. (A) Total WBC counts and (B) ANCs was determined in peripheral blood of WT and X-CGD mice 4 and 8 hours postperiodate injections (n > 9 in each group from ≥3 separate experiments). (C) Peritoneal neutrophils (Ly6G+, Ly6Cint) and (D) peritoneal monocytes (Ly6G−, Ly6Chi/int) were identified by flow cytometry in mice sacrificed 2 hours, 4 hours, 8 hours, or 24 hours postperiodate challenge. Data are presented as mean ± standard deviation and P values for each time point were determined using the Student t test. *P < .05, **P < .1, ***P < .001. Other mice were challenged i.p. with MSU or NC. (E) Peritoneal neutrophils and (F) monocytes were enumerated after 4 hours. Combined total of n = 6 mice in each group from 2 separate experiments. Data are presented as mean ± standard deviation and statistical differences calculated at each time point using the Student t test. *P < .05, **P < .01, ***P < .001. NC, necrotic cell lysate; WBC, white blood cell.
X-CGD mice exhibited enhanced response to sterile tissue injury. (A) Total WBC counts and (B) ANCs was determined in peripheral blood of WT and X-CGD mice 4 and 8 hours postperiodate injections (n > 9 in each group from ≥3 separate experiments). (C) Peritoneal neutrophils (Ly6G+, Ly6Cint) and (D) peritoneal monocytes (Ly6G−, Ly6Chi/int) were identified by flow cytometry in mice sacrificed 2 hours, 4 hours, 8 hours, or 24 hours postperiodate challenge. Data are presented as mean ± standard deviation and P values for each time point were determined using the Student t test. *P < .05, **P < .1, ***P < .001. Other mice were challenged i.p. with MSU or NC. (E) Peritoneal neutrophils and (F) monocytes were enumerated after 4 hours. Combined total of n = 6 mice in each group from 2 separate experiments. Data are presented as mean ± standard deviation and statistical differences calculated at each time point using the Student t test. *P < .05, **P < .01, ***P < .001. NC, necrotic cell lysate; WBC, white blood cell.
It is important to note that we saw no evidence in the X-CGD mice used in this study for proinflammatory polarization of peritoneal macrophages at baseline compared with WT mice (supplemental Figure 2). Analysis indicated similar expression profiles for IL-1α, -β, IL-6 as well as Ym1 and CD206; PPARγ expression, a marker associated with alternative activation, was even modestly increased in X-CGD macrophages (supplemental Figure 2). X-CGD mice also had similar spleen size, bone marrow cellularity, and content of neutrophils, granulocyte-monocyte progenitors, common myeloid progenitors, megakaryocyte/erythroid progenitors, and Lin−Sca+kit+ cells at baseline compared with WT mice (supplemental Figure 3).
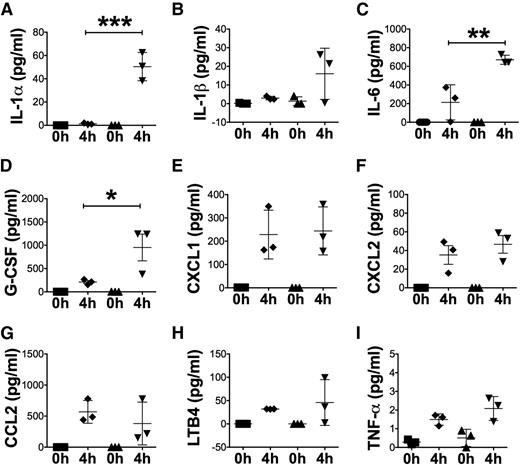
We hypothesized that differentially elevated inflammatory mediators following tissue injury were responsible for amplified acute-phase neutrophil mobilization and inflammation in X-CGD. Multiplex assay of peritoneal lavages 4 hours postperiodate injury revealed significantly elevated levels of IL-1α, IL-6, and G-CSF in X-CGD compared with WT mice (Figure 2A-D). Peritoneal IL-1β levels were elevated over naive mice but there were not significant differences between genotypes at 4 hours. Neutrophil chemoattractants, such as KC (CXC chemokine ligand 1 [CXCL1]), macrophage-inflammatory protein 2 (MIP2; CXCL2), and leukotriene B4 (LTB4), as well as the monocyte chemokine CC chemokine ligand 2 (CCL2), were also not differentially increased between the genotypes (Figure 2E-H). Finally, tumor necrosis factor-α (TNF-α) did not increase significantly in either WT or X-CGD mice in response to periodate-mediated peritoneal injury (Figure 2I).
Peritoneal IL-1α, IL-6, and G-CSF were significantly upregulated in X-CGD mice. (A-I) Multiplex cytokine array analysis of lavage fluid from peritoneal cavities in WT (▪) and X-CGD mice (□) obtained 4 hours postperidoate i.p. (H) Lavage fluid was also analyzed for LTB4 by ELISA. Data from 1 of 2 representative experiments with n = 3 mice per group are presented as mean ± standard deviation. Data were analyzed using 1-way ANOVA and adjusted P values after Tukey correction are shown. *P < .05, **P < .01, ***P < .001. ANOVA, analysis of variance.
Peritoneal IL-1α, IL-6, and G-CSF were significantly upregulated in X-CGD mice. (A-I) Multiplex cytokine array analysis of lavage fluid from peritoneal cavities in WT (▪) and X-CGD mice (□) obtained 4 hours postperidoate i.p. (H) Lavage fluid was also analyzed for LTB4 by ELISA. Data from 1 of 2 representative experiments with n = 3 mice per group are presented as mean ± standard deviation. Data were analyzed using 1-way ANOVA and adjusted P values after Tukey correction are shown. *P < .05, **P < .01, ***P < .001. ANOVA, analysis of variance.
As an approach to examine whether there were intrinsic differences in the ability of NADPH oxidase–positive and –negative leukocytes to accumulate in the peritoneum following periodate injury, we took advantage of X-CGD carrier females, where only ≈one-half of neutrophils and monocytes express CYBB because of X-linked inactivation. However, similar numbers of NADPH oxidase–positive and NADPH oxidase–negative populations were present in the peritoneum 4 hours after challenge with periodate (supplemental Figure 4). This indicates that the increased accumulation of peritoneal neutrophils during X-CGD peritonitis (Figure 1C) is not intrinsic to NADPH oxidase–deficient neutrophils.
We also compared neutrophil apoptosis and their ingestion by macrophages (efferocytosis) in X-CGD and WT mice. Apoptotic CGD neutrophils are ingested less efficiently than WT, reflecting impaired production of lysophosphatidylserine, which otherwise enhances efferocytosis.33 At 8 hours after the onset of inflammation (Figure 1C), <2% of neutrophils were apoptotic (data not shown). Thus, impaired efferocytosis is unlikely to account for the ≈twofold higher peritoneal neutrophils in X-CGD mice at this time. By 15 hours after periodate, peritoneal neutrophil numbers were declining (Figure 1C and not shown). At 15 hours, ≈20% of macrophages recovered from both WT and X-CGD peritonea contained an ingested neutrophil (supplemental Figure 5). The fraction of uningested apoptotic neutrophils in WT mice was ≈20%, but was significantly higher (≈1.5-fold) in X-CGD (supplemental Figure 5). This is consistent with less efficient uptake of apoptotic X-CGD neutrophils, which could contribute to their higher numbers at this phase of inflammation.
NADPH oxidase–deficient hematopoietic cells were required for increased IL-1α
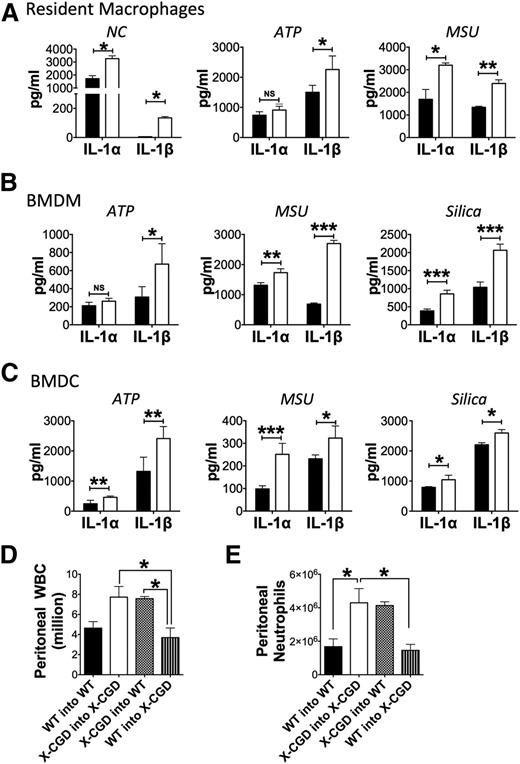
Previous studies implicate sentinel resident peritoneal macrophages as the primary source of IL-1α in sterile peritoneal inflammation induced by necrotic cells.24 As already mentioned, resident macrophages from naive WT and X-CGD mice expressed comparable levels of IL-1α and IL-1β transcripts (supplemental Figure 2) although lack IL-1α and IL-1β protein in cell lysates (data not shown). To determine whether periodate injury induced ROS, we harvested peritoneal cells 30 minutes after periodate, finding increased ROS production by WT but not X-CGD cells (supplemental Figure 5). However, it is difficult to subsequently recover resident peritoneal macrophages after the onset of acute inflammation as they strongly adhere to peritoneal membranes, a poorly understood phenomenon described as macrophage disappearance reaction.34 Thus, we performed in vitro studies to address whether X-CGD myeloid cells have a differential response to agonists inducing IL-1α. When challenged with necrotic lysates, X-CGD resident peritoneal macrophages released significantly higher amounts of both IL-1α and IL-1β, compared with WT cells (Figure 3A). Of note, much lower amounts of IL-1β were detected compared with IL-1α, consistent with in vivo peritoneal lavage profiles at 4 hours showing higher IL-1α levels compared with IL-1β (Figure 2). In addition, activators of IL-1 release elicited significantly higher secretion of both IL-1α and IL-1β by X-CGD resident macrophages, BMDMs, and BMDCs compared with WT cells (Figure 3A-C). Whereas LPS priming and subsequent challenge with DAMPs, ATP, or MSU crystals induced similar levels of IL-1α and IL-1β transcripts (supplemental Figure 7A), the content of pro- and cleaved forms of IL-1α and IL-1β was increased in X-CGD BMDCs compared with WT BMDCs following LPS priming and additional stimulation with ATP or MSU (supplemental Figure 7B). Stimulation of BMDCs with MSU crystals, a particulate DAMP, also induced NADPH oxidase–dependent ROS (supplemental Figure 7C). Taken together, these results demonstrate that NADPH oxidase–deficient murine myeloid cells have an intrinsically enhanced IL-1 response to a variety of agonists.
NADPH oxidase deficiency augmented IL-1 response. (A-C) Sorted resident peritoneal macrophages, BMDMs, and BMDCs from WT (▪) or X-CGD mice (□) were primed in vitro with ultrapure LPS prior to stimulation with necrotic EL4 lysates or inflammasome agonists, as indicated. IL-1α and IL-1β levels were determined by ELISA in cell-free supernatants. *P < .05, **P < .1, ***P < .001. Representative data from 1 of 3 experiments. For each experiment, 1 WT and 1 X-CGD mouse were used to isolate ResM or generate BMDMs and BMDCs. (D-E) Lethally irradiated recipient mice were transplanted with donor marrow to generate the following groups (donor into recipient): WT into WT, X-CGD into X-CGD, X-CGD into WT, and WT into X-CGD. Eight weeks after transplant, mice were challenged with periodate i.p. and peritoneal inflammation assessed 4 hours later by enumerating (D) total peritoneal WBCs and (E) neutrophils as determined by flow cytometry. Mean ± standard deviation is shown for 1 of 3 representative experiments with n = 3 per group. Differences for each cytokine (IL-1α or IL-1β) between genotypes were analyzed for every cell type and experimental condition separately and P values determined by the Student t test. *P < .05, **P < .01, ***P < .001. NC, necrotic cell lysate; ResM, sorted resident peritoneal macrophage.
NADPH oxidase deficiency augmented IL-1 response. (A-C) Sorted resident peritoneal macrophages, BMDMs, and BMDCs from WT (▪) or X-CGD mice (□) were primed in vitro with ultrapure LPS prior to stimulation with necrotic EL4 lysates or inflammasome agonists, as indicated. IL-1α and IL-1β levels were determined by ELISA in cell-free supernatants. *P < .05, **P < .1, ***P < .001. Representative data from 1 of 3 experiments. For each experiment, 1 WT and 1 X-CGD mouse were used to isolate ResM or generate BMDMs and BMDCs. (D-E) Lethally irradiated recipient mice were transplanted with donor marrow to generate the following groups (donor into recipient): WT into WT, X-CGD into X-CGD, X-CGD into WT, and WT into X-CGD. Eight weeks after transplant, mice were challenged with periodate i.p. and peritoneal inflammation assessed 4 hours later by enumerating (D) total peritoneal WBCs and (E) neutrophils as determined by flow cytometry. Mean ± standard deviation is shown for 1 of 3 representative experiments with n = 3 per group. Differences for each cytokine (IL-1α or IL-1β) between genotypes were analyzed for every cell type and experimental condition separately and P values determined by the Student t test. *P < .05, **P < .01, ***P < .001. NC, necrotic cell lysate; ResM, sorted resident peritoneal macrophage.
To provide evidence that sentinel macrophages are the key source of IL-1α in the response to periodate injury, we studied mice with a LysM-Cre–mediated deletion of the transcription factor GATA6 (GATA6Δmac mice), which have ≈twofold reduction in resident peritoneal macrophage numbers.27,35,36 Peritoneal IL-1α levels at 4 hours following periodate injury were similarly reduced in GATA6Δmac mice compared with GATA6flox/flox (supplemental Figure 8). However, the IL-1β responses were unaffected, suggesting that this isoform is derived from recruited exudate leukocytes. Together with the above studies of IL-1 release, these results implicate peritoneal resident macrophages as the source of IL-1α in the peritoneal cavity following periodate injury, a response that is excessive in X-CGD mice.
Because the CYBB-containing NADPH oxidase is also expressed in certain nonhematopoietic cells such as endothelial cells,37 we generated bone marrow chimeras following lethal irradiation to test whether the amplified neutrophil response in X-CGD mice following periodate-induced injury was regulated solely by NADPH oxidase–deficient hematopoietic cells. By 2 months posttransplant, resident peritoneal macrophages were ≈90% donor-derived (data not shown), as expected.38,39 In X-CGD mice transplanted with WT marrow, numbers of inflammatory peritoneal leukocytes, including neutrophils, were significantly reduced following periodate injection (Figure 3D-E). Conversely, transplanting X-CGD marrow into WT hosts resulted in exaggerated neutrophilic inflammation (Figure 3D-E). Thus, NADPH oxidase–deficient hematopoietic cells are necessary and sufficient to mediate the exacerbated neutrophilic inflammation in periodate-induced peritonitis.
Neutralizing IL-1α but not IL-1β abrogated the increased peritoneal G-CSF and neutrophil influx
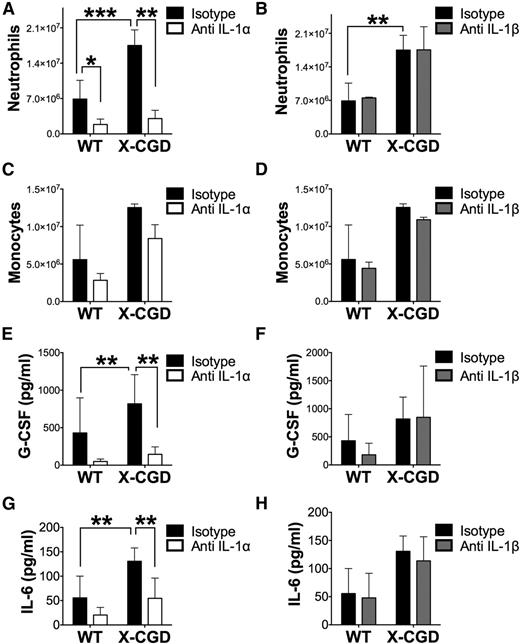
To determine whether IL-1α and IL-1β have nonredundant or overlapping roles in the response to periodate–induced peritonitis, we injected mice with neutralizing antibodies to either IL-1α or IL-1β just prior to induction of peritonitis as described.22 For both WT and X-CGD mice, neutralization of IL-1α but not IL-1β abrogated peritoneal neutrophil accumulation after 8 hours compared with isotype controls. However, inflammatory monocytes/macrophage numbers were not significantly affected by neutralization of either cytokine (Figure 4A-D). Notably, IL-1α but not IL-1β neutralization also ameliorated the increase in peritoneal G-CSF and IL-6 (Figure 4E-H). Using IL-1R−/− mice, we found that IL-1R signaling was essential for peritoneal neutrophil accumulation following periodate-induced injury (supplemental Figure 9A). Hence, these results demonstrate that IL-1α is the apical cytokine driving peritoneal neutrophilic inflammation following periodate injury and that neutralization of IL-1α is sufficient to ameliorate excessive peritoneal G-CSF and neutrophil exudation in X-CGD mice.
Neutralizing IL-1α, and not IL-1β, abrogated neutrophil early recruitment and increases in peritoneal G-CSF and IL-6. WT and X-CGD mice were injected IV with 0.5 mg of neutralizing antibodies either to mouse IL-1α, IL-1β, or isotype controls 1 hour before induction of periodate-induced peritonitis, and subsequently sacrificed after 8 hours. (A) Peritoneal neutrophils and (B) monocyte-macrophages were determined by cytospin analysis. Peritoneal cytokine levels in lavages were determined by a multiplex assay (E-H). Combined n = 6 in each group from 2 separate experiments. Data are presented as mean ± standard deviation and P values determined using 1-way ANOVA with Tukey correction. *P < .05, **P < .01, ***P < .001.
Neutralizing IL-1α, and not IL-1β, abrogated neutrophil early recruitment and increases in peritoneal G-CSF and IL-6. WT and X-CGD mice were injected IV with 0.5 mg of neutralizing antibodies either to mouse IL-1α, IL-1β, or isotype controls 1 hour before induction of periodate-induced peritonitis, and subsequently sacrificed after 8 hours. (A) Peritoneal neutrophils and (B) monocyte-macrophages were determined by cytospin analysis. Peritoneal cytokine levels in lavages were determined by a multiplex assay (E-H). Combined n = 6 in each group from 2 separate experiments. Data are presented as mean ± standard deviation and P values determined using 1-way ANOVA with Tukey correction. *P < .05, **P < .01, ***P < .001.
Prophylactic treatment with interferon-γ (IFN-γ) is reported to reduce bacterial and fungal infections in CGD and is frequently used as part of CGD management.1 It therefore was of interest to examine the impact of IFN-γ on the IL-1α/G-CSF axis and inflammation following periodate injury. However, IFN-γ treatment did not alter the acute-phase response in CGD mice, with similar peritoneal leukocyte counts and levels of IL-1α, G-CSF, and IL-6 in IFN-treated and saline control groups (supplemental Figure 10).
Acute peripheral neutrophilia and periodate-induced peritoneal inflammation was dependent on G-CSF
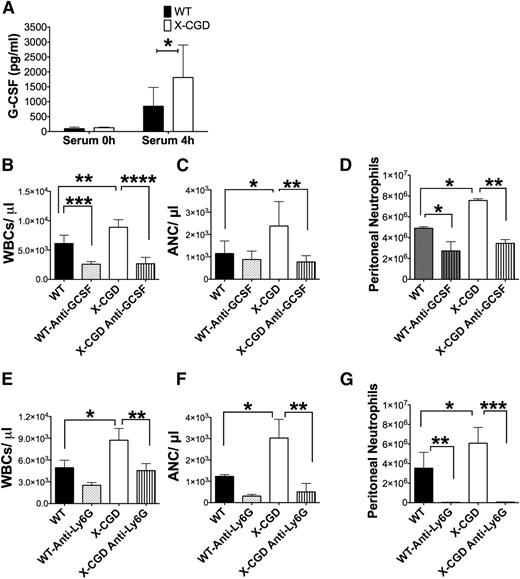
We next investigated the role of G-CSF (Figures 2A, 4E) in the rapid neutrophilic response to periodate-induced peritonitis (Figure 1B-C). Only a small fraction of the mature neutrophil pool is present in the periphery at homeostasis (in mice, <2%40 ). During inflammation, production of G-CSF and CXCL chemokines within inflamed tissues increases their levels in the bloodstream to induce neutrophil mobilization,13,14 which is critical for their rapid deployment. At 4 hours after peritoneal injury, serum levels of G-CSF were substantially elevated over baseline (Figure 5A). X-CGD mice had higher serum G-CSF levels compared with WT (Figure 5A), consistent with their higher peritoneal G-CSF levels (Figure 2D). Administration of a neutralizing antibody to G-CSF just prior to induction of periodate injury prevented the peripheral leukocytosis and neutrophilia in both WT and X-CGD mice (Figure 5B-C). In turn, reducing peripheral neutrophilia by inhibition of G-CSF significantly reduced neutrophil exudation into the peritoneal cavity (Figure 5D). Thus, enhanced numbers of circulating neutrophils directly correlated with greater tissue infiltration during periodate-induced inflammation. To further substantiate this point, WT mice were injected with G-CSF to mobilize neutrophils and then challenged with periodate after 2 hours. The G-CSF–mediated increase in peripheral neutrophilia augmented peritoneal recruitment of neutrophils (supplemental Figure 9B-C). These results establish that the periodate injury–induced increase in G-CSF mediated the peripheral neutrophilia and the magnitude of peritoneal neutrophil exudation, both of which were significantly enhanced in NADPH oxidase–deficient X-CGD mice.
Antibodies to G-CSF or Ly6G prevented neutrophil mobilization and subsequent recruitment to peritoneal cavity. (A) Serum levels of G-CSF were estimated by ELISA 4 hours postperiodate injections (n > 5). Data are presented as mean ± standard deviation was analyzed using the Mann-Whitney U test; *P < .05. (C-G) Mice were injected IV with neutralizing antibodies to mouse G-CSF or with anti-Ly6G to deplete neutrophils, then challenged with periodate and sacrificed after 8 hours. (B,E) WBC and (C,F) ANC in peripheral blood were determined by Hemavet analysis, and (D,G) peritoneal neutrophils enumerated by flow cytometry for anti-G-CSF–injected mice and by cytospins for anti-Ly6G–injected mice. For G-CSF antibody, n = 5 per group from 1 experiment; anti-Ly6G, n = 6 per group from 2 separate experiments. For B-G, data are presented as mean ± standard deviation and P values determined using 1-way ANOVA with Tukey correction. . *P < .05, **P < .01, ****P < .0001.
Antibodies to G-CSF or Ly6G prevented neutrophil mobilization and subsequent recruitment to peritoneal cavity. (A) Serum levels of G-CSF were estimated by ELISA 4 hours postperiodate injections (n > 5). Data are presented as mean ± standard deviation was analyzed using the Mann-Whitney U test; *P < .05. (C-G) Mice were injected IV with neutralizing antibodies to mouse G-CSF or with anti-Ly6G to deplete neutrophils, then challenged with periodate and sacrificed after 8 hours. (B,E) WBC and (C,F) ANC in peripheral blood were determined by Hemavet analysis, and (D,G) peritoneal neutrophils enumerated by flow cytometry for anti-G-CSF–injected mice and by cytospins for anti-Ly6G–injected mice. For G-CSF antibody, n = 5 per group from 1 experiment; anti-Ly6G, n = 6 per group from 2 separate experiments. For B-G, data are presented as mean ± standard deviation and P values determined using 1-way ANOVA with Tukey correction. . *P < .05, **P < .01, ****P < .0001.
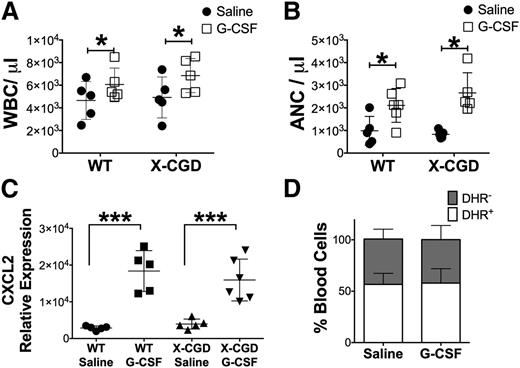
We also examined whether G-CSF–induced peripheral neutrophilia was itself regulated by the NADPH oxidase. However, administration of exogenous G-CSF elicited a similar increase in circulating neutrophils in X-CGD mice compared with WT (Figure 6A-B). G-CSF–induced neutrophil mobilization is in part mediated by increased marrow CXCL2 expression,13 which was also similar in WT and X-CGD mice (Figure 6C). We also examined G-CSF–induced neutrophil mobilization in X-CGD carrier females, who have, on average, equal numbers of NADPH oxidase–positive and –negative neutrophils. This fraction was unchanged following G-CSF (Figure 6D). These results indicate that NADPH oxidase deficiency does not enhance sensitivity to G-CSF–induced neutrophil mobilization, and that the increased neutrophilia in X-CGD mice (Figure 1B) in response to periodate-induced peritonitis reflects the higher G-CSF levels (Figure 5A).
NADPH oxidase deficiency did not result in intrinsic sensitivity to G-CSF. (A) Peripheral WBC and (B) ANC were determined 2 hours after IV injection with 5 μg of G-CSF or saline (n = 7). Data are presented as mean ± standard deviation. For panels A and B, P values were determined by analyzing the differences between control (Saline) and treated (G-CSF) experimental groups for each genotype by using the Student t test. *P < .05. (C) Cxcl2 expression was determined in total bone marrow 2 hours post-G-CSF injections. (D) Carrier females were injected with 5 μg of G-CSF and the relative fraction of oxidase-negative (DHR−) and oxidase-positive (DHR+) neutrophils determined by DHR assay. For panels C and D, data were analyzed using 1-way ANOVA with Tukey correction; ***P < .001 (C-D).
NADPH oxidase deficiency did not result in intrinsic sensitivity to G-CSF. (A) Peripheral WBC and (B) ANC were determined 2 hours after IV injection with 5 μg of G-CSF or saline (n = 7). Data are presented as mean ± standard deviation. For panels A and B, P values were determined by analyzing the differences between control (Saline) and treated (G-CSF) experimental groups for each genotype by using the Student t test. *P < .05. (C) Cxcl2 expression was determined in total bone marrow 2 hours post-G-CSF injections. (D) Carrier females were injected with 5 μg of G-CSF and the relative fraction of oxidase-negative (DHR−) and oxidase-positive (DHR+) neutrophils determined by DHR assay. For panels C and D, data were analyzed using 1-way ANOVA with Tukey correction; ***P < .001 (C-D).
Reducing early neutrophilic response influx promotes resolution of peritoneal inflammation in X-CGD
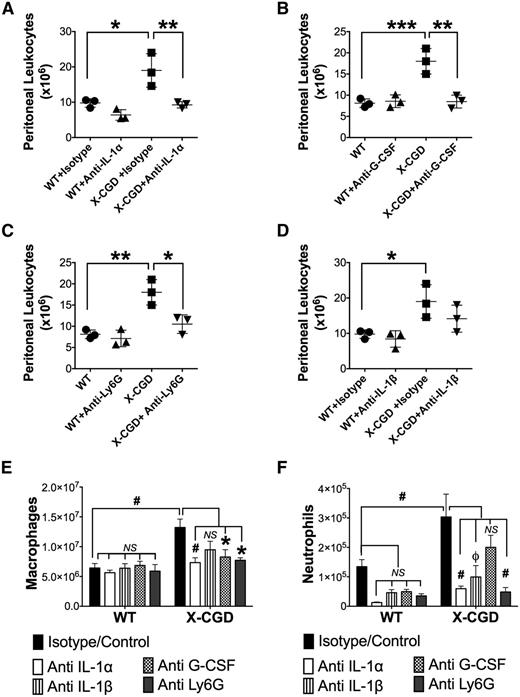
X-CGD mice have delayed resolution of periodate-induced peritoneal inflammation,28 and we hypothesized that blocking the IL-1/G-CSF axis to attenuate the excessive early neutrophilic response would reduce inflammation at later time points. At 72 hours, following periodate injection, peritoneal leukocytes in X-CGD mice were still threefold higher than in naive mice, whereas these were close to baseline in WT mice (Figure 7; supplemental Figure 11). Macrophages, almost all of which were inflammatory F4/80int monocyte-derived macrophages,28 were the predominant leukocyte in WT and X-CGD peritonea at 72 hours (Figure 7E; supplemental Figure 11), although some neutrophils were also present (Figure 7F); both inflammatory macrophages and neutrophils were present in higher numbers in X-CGD compared with WT. Peritoneal G-CSF levels at 72 hours were much reduced compared with 8 hours, but remained significantly elevated in X-CGD mice (supplemental Figure 12). Early neutralization of either IL-1α or G-CSF resulted in a significant reduction in inflammatory peritoneal leukocytes in X-CGD to numbers similar to WT mice (Figure 7A-B). Importantly, this reduction reflected lower numbers of both exudate macrophages and neutrophils (Figure 7E-F). Although early neutralization of IL-1β had no affect on acute inflammation (Figure 4), this produced a significant reduction in neutrophils at 72 hours in both WT and X-CGD mice (Figure 7F), suggesting that IL-1β contributes to the later phase of inflammation independent of IL-1α.
Blocking the early neutrophil response to sterile inflammation protected against prolonged elevation of peritoneal exudate cells in X-CGD. (A-D) WT and X-CGD mice were injected IV with neutralizing antibodies to either mouse IL-1α, G-CSF, IL-1β, or isotype control antibody, or with anti-Ly6G to deplete neutrophils, then challenged 1 hour with periodate i.p. and sacrificed after 72 hours. Total peritoneal leukocyte counts were enumerated. Data from 1 of 2 representative experiments (n = 3 mice per group) is presented as means ± standard deviation and statistical differences calculated using 1-way ANOVA with Tukey correction. *P < .05, **P < .01, ***P < .001. (E-F) Total number of macrophages (F4/80+) and neutrophils (Ly6G+) in the peritoneal exudates at 72 hours are shown. Each antibody-treated group was compared with a combined group of mice injected with periodate alone or with isotype + periodate as controls (▪). Statistical differences between controls and respective treatment groups were calculated using 1-way ANOVA with Dunnett correction. *P < .05, ϕP < .01, #P < .001; NS, nonsignificant.
Blocking the early neutrophil response to sterile inflammation protected against prolonged elevation of peritoneal exudate cells in X-CGD. (A-D) WT and X-CGD mice were injected IV with neutralizing antibodies to either mouse IL-1α, G-CSF, IL-1β, or isotype control antibody, or with anti-Ly6G to deplete neutrophils, then challenged 1 hour with periodate i.p. and sacrificed after 72 hours. Total peritoneal leukocyte counts were enumerated. Data from 1 of 2 representative experiments (n = 3 mice per group) is presented as means ± standard deviation and statistical differences calculated using 1-way ANOVA with Tukey correction. *P < .05, **P < .01, ***P < .001. (E-F) Total number of macrophages (F4/80+) and neutrophils (Ly6G+) in the peritoneal exudates at 72 hours are shown. Each antibody-treated group was compared with a combined group of mice injected with periodate alone or with isotype + periodate as controls (▪). Statistical differences between controls and respective treatment groups were calculated using 1-way ANOVA with Dunnett correction. *P < .05, ϕP < .01, #P < .001; NS, nonsignificant.
We next examined the impact of reducing the early neutrophilic response to periodate inflammation by administering an anti-Ly6G antibody to deplete neutrophils30 just prior to injection with periodate. We first showed that anti-Ly6G markedly reduced peripheral blood neutrophils and completely abrogated peritoneal accumulation of neutrophils in both WT and X-CGD mice during the acute phase of periodate-induced inflammation (Figure 5E-G). Strikingly, this early depletion of neutrophils by anti-Ly6G also promoted resolution in X-CGD, as shown by reduced numbers of inflammatory macrophages and neutrophils in the peritoneal cavity at 72 hours (Figure 7C,E-F).
Discussion
The leukocyte NADPH oxidase is implicated in regulating multiple cellular and immune processes by modulation of redox-sensitive cellular proteins or by direct effects of derivative ROS, thus extending its role far beyond microbial killing.4,9,28,41 Chronic inflammatory complications as well as autoimmune manifestations are associated with CGD, and hypomorphic phox alleles are linked to autoimmunity and inflammatory bowel disease.1,7-10 How NADPH oxidase deficiency contributes to these disorders is incompletely understood. Prior studies in murine CGD have focused on pathogen or pathogen-dependent molecular pattern (PAMP)-dependent models to demonstrate hyperinflammatory responses in CGD, which have been associated with amplified nuclear factor-κB (NF-κB) activation, elevated proinflammatory cytokines such as TNFα, IL-6 and IL-1β, and often increased tissue neutrophils.4,42-44 However, whether the NADPH oxidase influences host responses to endogenous DAMPs was unknown.
Here, we identified sequential steps in an inflammatory cascade that were dysregulated in NADPH oxidase–deficient mice to mediate excessive acute-phase neutrophilic inflammation in response to sterile tissue injury, and showed that IL-1α has a nonoverlapping role with IL-1β. Prior studies showed that IL-1α production by sentinel macrophages drives the neutrophilic response to inflammation induced by necrotic cells.22,24 In CGD, elevated production of IL-1α following periodate-induced tissue injury increased G-CSF–induced neutrophilia that exacerbated inflammation. We also showed that X-CGD resident macrophages released excessive IL-1α in response to DAMPs in vitro, and marrow transplantation studies showed that NADPH oxidase–deficient hematopoietic cells were necessary and sufficient for the exaggerated response in vivo. Finally, blocking the excessive early neutrophil response by neutralization of IL-1α or G-CSF reduced inflammatory burden and promoted resolution, with subsequent reduced numbers of inflammatory monocyte-macrophages. This is consistent with studies demonstrating positive feedback between early neutrophil influx and recruitment of inflammatory monocytes, which can be mediated by a variety of neutrophil products.45 Although other factors contributing to delayed resolution of inflammation in NADPH oxidase deficiency remain to be investigated, such as proresolving lipid mediators,46 the current findings establish a link between prolonged inflammation and the amplified acute neutrophil recruitment to injured tissue via an upregulated IL-1α/G-CSF axis.
Our observations confirm and extend prior reports that NADPH oxidase–deficient myeloid cells have increased IL-1 release following stimulation. Human CGD monocytes produced elevated levels of IL-1β when stimulated in vitro with particulate and soluble activators of caspase-1 and NLRP3 inflammasome,44,47,48 and 1 study reported increased IL-1α release by LPS-stimulated human CGD monocytes.47 Here, we showed that NADPH oxidase deficiency augmented IL-1α and IL-1β release from murine resident macrophages, BMDMs, and BMDCs challenged with classic DAMPs that activate the NLRP3 inflammasome. However, we found that IL-1β played little role in vivo in driving excessive acute-phase inflammation in response to DAMP-associated tissue injury in CGD. Regulation of IL-1α biosynthesis, its release from the cell, and enhanced activation based on its cleavage are complex and only partially understood.17,19,20 Whereas we observed a similar induction of IL-1α transcripts in DAMP-activated WT and X-CGD myeloid cells, IL-1α protein levels were increased to a greater extent in X-CGD BMDCs. This suggests that posttranscriptional mechanisms could be involved in enhancing the IL-1α response in NAPDH oxidase deficiency. IL-1α release also appeared particularly augmented in X-CGD myeloid cells in response to necrotic cells and to MSU crystals and silica. Interestingly, these agonists are reported to induce IL-1α release in an inflammasome-independent manner both in vitro and in vivo.19
IL-1α is highly associated with neutrophilic inflammation, and this function, in many settings, is not redundant with IL-1β. This includes neutrophil recruitment in response to necrotic cells,22 chronic obstructive pulmonary disease (COPD),49 hypoxic tissue injury,50 atherosclerosis,51 neutrophilic dermatosis secondary to a mutant Src homology region 2 domain-containing phosphatase-1 phosphatase,52 adenovirus-induced splenic inflammation,53,54 and Legionella pneumophila pneumonia.55 We show that the mechanistic link between IL-1α and neutrophil extravasation in response to DAMP-associated tissue injury is mediated by IL-1α–induced production of G-CSF, which acts systemically to rapidly mobilize neutrophils from the marrow storage pool into circulation.56 Similarly, previous reports showed that IL-1α–dependent G-CSF response drove acute neutrophilia in alum-based inflammation as well as in a model of neutrophilic dermatosis.52,57 This pathway is significantly enhanced in X-CGD.
G-CSF is a pleiotropic cytokine produced by nonhematopoietic cells (stromal, endothelial, fibroblasts) and hematopoietic cells (neutrophils, macrophages) and its production is rapidly induced by IL-1, TNF-α, and other inflammatory stimuli.58 Human and mouse peritoneal mesothelial cells can produce G-CSF and other cytokines downstream of IL-1R signaling.23,59 G-CSF levels increased rapidly in inflamed peritoneal tissue (for example, following i.p. casein injection, which resulted in subsequent increased serum G-CSF and neutrophil mobilization56 ) and peritoneal injection of anti-G-CSF antibody just prior to thioglycollate challenge prevented subsequent neutrophil mobilization.14
This study provides additional insights into how NADPH oxidase deficiency can promote neutrophilic inflammation. Prior studies demonstrated impaired macrophage clearance of apoptotic neutrophils in NADPH oxidase deficiency, reflecting in part deficient generation of lysophosphatidylserine, which enhances their uptake by macrophages, and in part decreased macrophage efferocytosis.60-62 We also observed a higher fraction of uningested apoptotic neutrophils, at a time when peritoneal neutrophil counts were falling, although a similar fraction of efferocytosing macrophages in WT and X-CGD mice. However, our results suggest that the predominant mechanism underlying increased neutrophilic inflammation in DAMP-associated injury in CGD is amplified IL-1α/G-CSF production, leading to an enhanced systemic response that delivers increased numbers of neutrophils to the peritoneum; treatments that normalized excessive early neutrophil accumulation to WT levels were sufficient to promote resolution at a later stage.
In conclusion, we demonstrate that the NADPH oxidase acts to restrain the IL-1α/G-CSF axis that initiates the systemic neutrophil response to sterile tissue injury. Together with prior studies showing enhanced release of IL-1β by CGD myeloid cells in other settings, excessive IL-1 may contribute to pathologic inflammatory responses in CGD, and targeting IL-1 could be an approach to alleviating inflammatory complications in these patients. Indeed, several small studies reported that administration of the IL-1R antagonist Anakinra to CGD patients with colitis improved symptoms. Finally, although IL-1α and IL-1β share overlapping downstream signaling pathways, the results of the current study reinforce recent evidence that these 2 cytokines have nonredundant roles in regulating inflammation. Therapeutic agents that selectively target each of these cytokines are now available and in clinical trials for other disorders. It is therefore important to understand the nuances of how IL-1α and IL-1β each regulate different types of inflammation in the context of NADPH oxidase deficiency.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Robyn Klein for IL1R−/− mice, Jesse Williams for help with genotyping Gata6 mutant mice, and Tina McGrath for assistance with manuscript preparation. The authors also thank Dr Wei Yang for helping with the statistical analyses in the manuscript.
This work was supported by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital grant (M.C.D.) and National Institutes of Health, National Institute of Allergy and Infectious Diseases grant AI049653 (G.J.R.).
Authorship
Contribution: J.B., N.K.P., A.A., and M.C.D. designed research; J.B., N.K.P., S.I., M.Y.Z., A.A., S.P., and G.H. performed and/or analyzed experiments; J.B. prepared the figures; and J.B. and M.C.D. wrote the manuscript with input from S.I., M.Y.Z., and G.J.R.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary C. Dinauer, Washington University School of Medicine in St. Louis, 660 S. Euclid Ave, PO Box 8208, St. Louis, MO 63110; e-mail: dinauer_m@kids.wustl.edu.