Abstract
Background: Patients (pts) with CLL harboring 17p deletion [del(17p)] are considered to have very poor prognosis. Venetoclax (VEN) is an orally bioavailable, selective BCL-2 inhibitor that induces apoptosis in CLL cells independent of p53. A phase 1 study of VEN showed high response rates in pts with relapsed/refractory (R/R) CLL, including del(17p) CLL (overall response rate, ORR = 77%). This pivotal phase 2, single-arm, multicenter study evaluated VEN monotherapy in pts with R/R del(17p) CLL.
Methods: Pts with R/R del(17p) CLL, assessed in peripheral blood (PB) by a central laboratory ( >7% cells by Vysis FISH probe), commenced VEN once daily with a weekly dose ramp-up schedule (20, 50, 100, 200, 400 mg) over a period of 5 weeks with tumor lysis syndrome (TLS) prophylaxis. Pts were treated with daily 400 mg continuous dosing until disease progression or discontinuation for another reason. The primary objective was to determine the ORR. Responses were determined by both an independent review committee (IRC) and investigators using iwCLL 2008 criteria. Efficacy analyses were pre-specified to occur once pts completed 36 weeks of VEN, had disease progression, or permanently discontinued. Secondary objectives included CR and PR rates, time to first response, duration of response (DoR), progression-free survival (PFS), overall survival (OS), the proportion of pts proceeding to allogeneic stem cell transplant (allo-SCT), and safety. The level of minimal residual disease (MRD) in PB and/or bone marrow (BM) was assessed in a subset of pts by multi-color flow cytometry using iwCLL-recommended sensitivity criteria of <10-4.
Results: A total of 107 pts were enrolled in the main cohort (June 2013–June 2014). Median (range) age was 67 (37–85) years; 65% were male. Median number of prior regimens was 2 (1–10); 78 pts (72.9%) had received prior fludarabine (F), 34 (37.4%) were F-refractory, 54 (50.5%) received prior bendamustine (B), and 27 (50%) were B-refractory. 45 pts (42.1%) were high-risk for TLS based on lymph nodes ≥10 cm (or nodes ≥5 cm with ALC ≥25x109/L). All pts but one had del(17p); 60 of 83 pts with available data (72.3%) had mutated TP53 (investigator reported). As of the data cut-off (April 30, 2015), the median time on study was 12.1 (0.03–21.5) months.
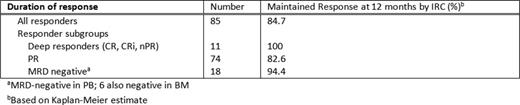
The primary endpoint of IRC-assessed ORR was 79.4% (95% CI: 70.5%–86.6%). Deep responses included 7.5% CR/CRi and 2.8% nPR, by IRC (Table). Among pts who achieved PR (69.2%, excluding nPR) or non-responders (20.6%) by IRC, 17 pts (15.9%) had no morphological evidence of CLL in the BM. Investigator-assessed ORR is also reported in the Table. 45 pts had an MRD assessment. Notably 18 pts (17% of whole cohort, 21% of responders) had no detectable MRD in the PB; 10 of these were also tested in BM, 6 were MRD-negative.
Median time-to-first response was 0.8 months (0.1–8.1); median time to CR/CRi was 8.2 months (3.0–16.3) by IRC. Overall median DoR, PFS, and OS were not reached. The actuarial 12-month PFS and OS rates were 72.0% and 86.7%, respectively (actuarial 12-month DoR rates in Table).
37 pts discontinued treatment: 22 due to PD (9 Richter's transformation), 9 due to AE, 2 withdrew consent, and 1 with non-compliance; 3 pts proceeded to allo-SCT (2 PR, 1 CR by IRC at time of transplant). 11 deaths occurred (≤30 days from last dose of VEN): 7 due to PD, 4 due to AE (stroke, liver derangement, septic shock, and cardio-respiratory insufficiency). 7 additional deaths occurred beyond 30 days from VEN discontinuation (39-328 days) due to PD.
Treatment-emergent AEs (all grades) in ≥20% pts were neutropenia (43%), diarrhea (29%), nausea (29%), anemia (27%), and fatigue (22%). Grade 3/4 AEs in ≥10% pts were neutropenia (40%; 25 pts had grade 4), anemia (18%), and thrombocytopenia (15%). 22.4% of pts had neutropenia (any grade) at study entry. Infection ≥ grade 3 occurred in 20% of pts; most common was pneumonia (5%). Laboratory TLS was reported in 5 pts; none had clinical consequences, and all were manageable with electrolyte management and 1-day dose interruption (2 pts).
Stilgenbauer:AbbVie, Amgen, Boehringer-Ingelheim, Celgene, Genentech, Genzyme, Gilead, GSK, Janssen, Mundipharma, Novartis, Pharmacyclics, Hoffman La Roche: Honoraria , Membership on an entity’s Board of Directors or advisory committees , Research Funding. Off Label Use: Venetoclax is an investigational drug and has no label at this time. Eichhorst:AbbVie, Roche: Membership on an entity’s Board of Directors or advisory committees , Research Funding , Speakers Bureau. Schetelig:GSK, Sanofi, Janssen, Neovii: Membership on an entity’s Board of Directors or advisory committees , Research Funding. Coutre:AbbVie: Research Funding ; Gilead: Research Funding ; Pharmacyclics LLC, an AbbVie Company: Consultancy , Membership on an entity’s Board of Directors or advisory committees , Research Funding ; Janssen: Consultancy , Honoraria , Membership on an entity’s Board of Directors or advisory committees ; Celgene Corporation: Membership on an entity’s Board of Directors or advisory committees , Research Funding ; Celgene Business Advisory Board: Membership on an entity’s Board of Directors or advisory committees ; Novartis: Research Funding. Seymour:Gilead: Honoraria , Membership on an entity’s Board of Directors or advisory committees ; Genentech, Inc.: Membership on an entity’s Board of Directors or advisory committees ; Celgene: Consultancy , Honoraria , Membership on an entity’s Board of Directors or advisory committees , Other: Travel support , Speakers Bureau ; Incyte: Honoraria , Membership on an entity’s Board of Directors or advisory committees ; AbbVie: Consultancy , Honoraria , Membership on an entity’s Board of Directors or advisory committees , Other: Travel support , Research Funding , Speakers Bureau ; Janssen: Honoraria , Membership on an entity’s Board of Directors or advisory committees , Research Funding ; Phebra: Consultancy , Honoraria , Membership on an entity’s Board of Directors or advisory committees ; Takeda: Honoraria , Membership on an entity’s Board of Directors or advisory committees ; Roche: Consultancy , Honoraria , Membership on an entity’s Board of Directors or advisory committees , Other: Travel support , Research Funding ; Infinity: Honoraria , Membership on an entity’s Board of Directors or advisory committees. Puvvada:Genentech, AbbVie, Spectrum, Janssen and Takeda, Pharmacyclics, Genentech/Roche: Consultancy , Membership on an entity’s Board of Directors or advisory committees , Other: Travel funding for Investigators meeting , Research Funding. Wendtner:Mundipharma: Consultancy , Other: travel grants , Research Funding ; Celege: Consultancy , Other: Travel grants , Research Funding ; Gilead: Consultancy , Other: travel grants , Research Funding ; Glaxo-SmithKline: Consultancy , Other: travel grants , Research Funding ; Janssen-Cilag: Consultancy , Other: travel grants , Research Funding ; Pharmacyclics: Consultancy , Other: travel grants , Research Funding ; Hoffmann-LaRoche: Consultancy , Other: travel grants , Research Funding ; Genentech: Consultancy , Other: travel grants , Research Funding ; AbbVie: Consultancy , Other: travel grants , Research Funding. Roberts:AbbVie and Genentech: Research Funding ; Walter and Eliza Hall Institute of Medical Research: Employment. Jurczak:CELLTRION, Inc,: Research Funding ; Celgene, Eisai, Gilead, Janssen, Mundipharma, Pharmacyclics, Pfizer, Roche, Sandoz –Novartis, Spectrum, Takeda, AbbVie, Morphosys, Janssen, Mundipharma, Sandoz –Novartis, Spectrum, Takeda, Teva, Morphosys: Membership on an entity’s Board of Directors or advisory committees , Research Funding. Mulligan:Sanofi Aventis: Research Funding ; Celgene: Consultancy , Honoraria ; Roche: Consultancy , Honoraria , Research Funding , Speakers Bureau ; Janssen: Consultancy , Honoraria , Speakers Bureau ; AbbVie: Membership on an entity’s Board of Directors or advisory committees. Boettcher:AbbVie: Honoraria , Research Funding ; Celgene: Research Funding ; Roche: Honoraria , Other: travel grants , Research Funding. Mobasher:Genentech, Inc.: Employment ; Roche: Equity Ownership. Zhu:AbbVie: Employment , Equity Ownership. Chyla:AbbVie: Employment , Equity Ownership. Verdugo:AbbVie: Employment , Equity Ownership. Enschede:AbbVie: Employment , Equity Ownership. Cerri:AbbVie: Employment , Equity Ownership. Humerickhouse:AbbVie: Employment , Equity Ownership. Gordon:AbbVie: Employment , Equity Ownership. Hallek:GSK, Genentech: Membership on an entity’s Board of Directors or advisory committees , Research Funding ; Celgene: Honoraria , Other: Speakers Bureau and/or Advisory Boards , Research Funding ; Janssen: Honoraria , Other: Speakers Bureau and/or Advisory Boards , Research Funding ; Mundipharma: Honoraria , Other: Speakers Bureau and/or Advisory Boards , Research Funding ; Boehringher Ingelheim: Honoraria , Other: Speakers Bureau and/or Advisory Boards ; Pharmacyclics: Honoraria , Other: Speakers Bureau and/or Advisory Boards , Research Funding ; Roche: Honoraria , Other: Speakers Bureau and/or Advisory Boards , Research Funding ; Gilead: Honoraria , Other: Speakers Bureau and/or Advisory Boards , Research Funding ; AbbVie: Honoraria , Other: Speakers Bureau and/or Advisory Boards , Research Funding. Wierda:AbbVie, Genentech: Consultancy , Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal