Abstract
Acute Graft-vs.-Host Disease (aGVHD) is the major cause of mortality and morbidity following allogeneic hematopoietic cell transplantation (AHCT). Steroids are the standard initial therapy yet only ~50-60% will achieve a response. Strategies to improve response and minimize steroid-exposure are needed. We report the results of a randomized trial of extracorporeal photopheresis (ECP) plus steroids vs. steroids- alone as initial therapy for aGVHD.
Methods: This was a Phase II, randomized, adaptive Bayesian design. Patients with histologically confirmed aGVHD and who received <72 hours of steroids were randomized to receive 2mg/kg methylprednisolone (MP) with or without ECP. ECP schedule was: days 1-14 (8 sessions), days 15-28 (6 sessions) and days 29-56 (8 sessions). The primary endpoint was treatment success at day 56 post-randomization defined as being alive, in remission, achieving a GVHD response without additional therapy, and on < 1mg/kg of MP at day 28 and < 0.5mg/kg on day 56. Randomization probabilities were based upon the probabilities of response in each arm, stratified by skin-only vs. visceral involvement. The primary endpoint was evaluated using a Bayesian predictive probability approach; i.e., at the end of the study, if the posterior probability that the success rate for either arm was greater than that for the other arm was > 0.80, that arm would be declared the preferred treatment.
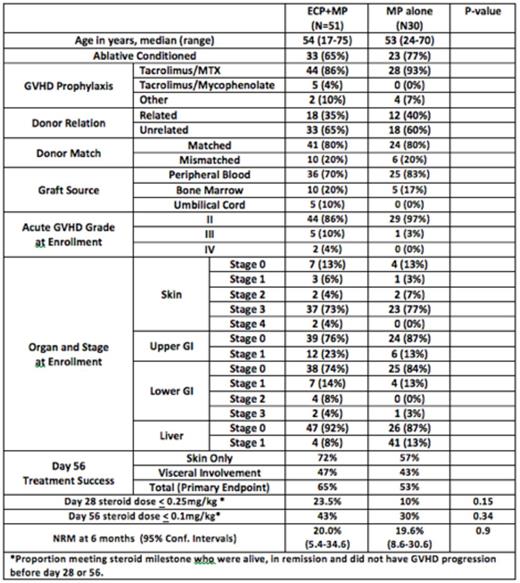
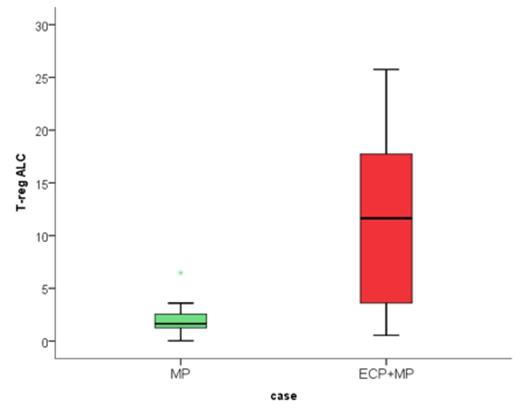
Pts and Results: Between 2008 and 2014 eighty-one (81) pts were randomized to ECP + MP (51 pts) or MP-alone (30 pts). Median age was 54 yrs (range, 17-75); 42% had AML/MDS and 62% were male. HCT was performed with myeloablative conditioning in 69% and 63% had an unrelated donor. At enrollment, 90% of patients had aGVHD grade II, 10% had grade III/IV. Organ Involvement was: skin (86%), upper GI (22%), lower GI (22%) and liver (10%). The treatment arms were balanced for all baseline characteristics. The probability that ECP+MP had a higher success rate than MP-alone was 0.815 which exceeded the protocol-specified threshold of 80%, declaring ECP-arm the preferred treatment. The ECP-arm was more beneficial in pts with skin-only aGVHD (72% vs. 57% response rate) whereas visceral-organ involvement response rates were similar (47% vs. 43%). By day 56, 43% of the evaluable patients randomized to the ECP were on physiologic doses of steroids (≤0.1mg/kg) versus 30% for MP-alone arm (p=0.34). To study the immunologic effects of ECP, we examined the recovery of T-cell subsets by multi-parameter flow cytometry in a subset of pts randomized to the ECP+MP and MP-alone arm at enrollment and study conclusion. ECP+MP was associated with more robust recovery of CD4+ and CD8+ cells and significantly higher absolute number of regulatory T-cells (p=0.043), figure 1.
Conclusions: The results of this randomized, phase II trial indicate that the addition of ECP to steroids results in higher GVHD response and facilitates steroid-tapering in pts with newly diagnosed aGVHD. This combination appears most efficacious for pts with skin-involvement. Correlative studies implicate expansion of regulatory T-cells as a possible mechanism of action through which ECP exerts its immunologic effect.
Mean Absolute Regulatory T-cell Count in subset of patients randomized to ECP+MP (red) vs. MP-alone (green) p-value= 0.043.
Mean Absolute Regulatory T-cell Count in subset of patients randomized to ECP+MP (red) vs. MP-alone (green) p-value= 0.043.
Alousi:Therakos, Inc: Research Funding. Off Label Use: Extracorporeal Photopheresis is FDA approved for the treatment of cutaneous lymphoma. It is commonly used in the treatment of patients with acute and chronic graft-versus-host disease. There are no FDA approved therapies for this indication and all therapies are given as an "off-label" indication..
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal