Abstract
Introduction: Early ASCT improves progression-free survival (PFS) but not overall survival (OS) in poor prognosis DLBCL treated with R-CHOP immuno-chemotherapy (Stiff P et al., NEJM 2013). Furthermore, indiscriminate ASCT exposes those poor-risk patients destined to remain in CR with R-CHOP alone to unnecessary toxicity. Here, the role of interim PET/CT to risk-stratify is controversial. One confounding factor is that interim PET/CT is typically performed after 2 (or 3) cycles of chemotherapy when FDG-avidity likely reflects a mix of residual cancer cells and inflammation; in contrast, interim PET/CT after cycle 4 may be more representative of resistant lymphoma. We hypothesized that: 1. assessment of DLBCL patients by interim PET/CT after 4 cycles (iPET4) of R-CHOP-14 allows greater specificity of FDG-avidity; and, 2. for patients with iPET4-positivity, early treatment intensification with R-ICE chemotherapy followed by Zevalin-BEAM conditioning and ASCT results in 2-yr PFS that is equivalent to interim-PET/CT-negative patients treated with R-CHOP alone.
Methods: We conducted a prospective multicenter phase 2 study which enrolled DLBCL patients ≤ 70 yrs with either IPI=2-5, or IPI =0-1 with tumor bulk (≥ 7.5 cm), and who were considered fit for ASCT. All patients underwent a diagnostic PET/CT at study entry and were planned to receive 4 cycles of R-CHOP-14 and supported with Pegfilgrastim. Cycle 5 of R-CHOP-14 was delayed 7 days, and an interim PET/CT scan was undertaken at d17-d20 post-4th R-CHOP-14, the delay intended to reduce the impact of rebound inflammation after R-CHOP. All interim PET/CT scans were assessed centrally by a core group of imaging specialists using International Harmonization Project (IHP) criteria with mediastinal blood pool as the reference. Biopsy to confirm residual tumor was not mandated. iPET4-positive patients received 3 cycles of R-ICE followed by ASCT with Zevalin-BEAM conditioning; those who were iPET4-negative received a further 2 cycles of R-CHOP-14 plus 2 doses of Rituximab.
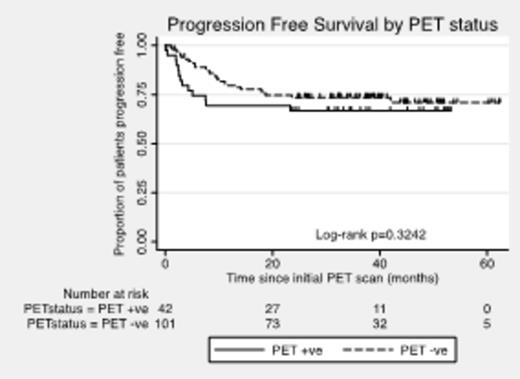
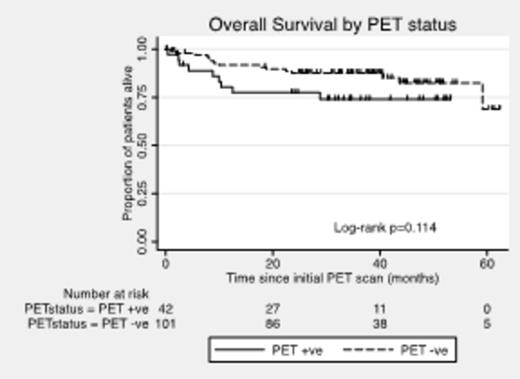
Results: A total of 162 patients were enrolled from 20 Australian centers; 11 patients were excluded because of failure to meet inclusion criteria. Baseline characteristics of the 151 evaluable patients included: median age 57 yrs (range, 21 to 69), 40% aged > 60 yrs, 62% males, 79% stages 3 or 4, 13% ECOG PS > 1, 78% elevated LDH, 48% extranodal sites > 1, 54% bulky disease ≥ 7.5 cm, 20% IPI=0-1, 27% IPI=2, 31% IPI=3, and 23% IPI=4-5. No interim PET/CT scan was performed in 8 patients due to progressive disease (PD) (1), bowel perforation (2), toxicity (3), and dose delays ≥ 2 weeks (2). Interim PET/CT scans were undertaken at d17-d20 in 62% and d14-d16 in another 23%. Of the 143 patients with interim PET/CT, 101 (71%) were deemed iPET4-negative and 42 (29%) were iPET4-positive. Interestingly, the baseline characteristics were comparable between iPET4-negative and iPET4-positive patients. Of the 101 iPET4-negative patients, 5 failed to complete therapy due to PD (3), toxicity (1), or infection (1). Of the 42 iPET4-positive patients, 10 failed to complete intensification therapy due to PD (6), 2nd malignancy (1), or consent withdrawal (3). Among iPET4-positive patients undergoing ASCT, there was 1 treatment-related death due to viral infection. At a median follow-up of 35 months, the Kaplan-Meier (KM) estimate of 2-yr PFS and OS for the entire eligible cohort of 151 pts was 72% and 85%, respectively. For the 101 iPET4-negative and 42 iPET4-positive patients, 2-yr PFS was 74% and 67% (P =0.32) (Figure 1), and 2-yr OS was 88% and 78% (P=0.11) (Figure 2), respectively. Among iPET4-negative and iPET4-positive patients with IPI=3-5, 2-yr PFS was 65% and 69% (P=0.74), and 2-yr OS was 81% and 83% (P =0.85), respectively.
Conclusions: Patients with poor risk DLBCL who are interim PET/CT-positive after 4 cycles of R-CHOP-14 and switched to intensification with R-ICE followed by ASCT with Zevalin-BEAM have favorable rates of PFS and OS that are equivalent to that seen for patients who are interim PET/CT-negative. This study supports further investigation of treatment intensification in patients with poor risk DLBCL who are interim PET/CT-positive after 4 cycles of immuno-chemotherapy.
The study was supported in part by Roche Products Pty. Ltd, Amgen Australia Pty. Ltd, and Bayer Australia Ltd.
Hertzberg:Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Gill:AbbVie: Honoraria; Roche: Research Funding; Sanofi Aventis: Research Funding; Roche: Honoraria. Ho:Celgene: Other: Travel. Cull:Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Amgen: Other: Travel. Grigg:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lewis:Amgen: Other: Travel; Roche: Honoraria, Other: Travel. Renwick:Amgen: Other: Travel; Bayer: Speakers Bureau. Seymour:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech, Inc.: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Infinity: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Phebra: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal