Abstract
Background: Fludarabine plus busulfan (FB) and fludarabine plus melphalan (FM) are two commonly used reduced intensity conditioning (RIC) regimens for allogeneic hematopoietic stem cell transplantation (SCT). A recent analysis showed that both regimens had similar overall survival (OS) but relapse incidence (RI) was significantly higher with FB with a trend towards higher non-relapse mortality (NRM) in FM (Baron et al., Cancer 2015). However, that cohort included patients treated with oral and intravenous busulfan without pharmakokinetic targeting of intravenous which may impact anti-leukemic activity.
Aim: Compare transplant related outcomes of FB vs. FM using intravenous (IV) busulfan targeted to the area under the curve (AUC).
Methods: After due IRB approval, patients with acute myelogenous leukemia (AML) and myelodysplastic syndromes (MDS) receiving RIC-SCT from 2008-2014 were identified. All baseline and laboratory features were retrospectively extracted. Busulfan dose in FB was 0.8 mg/kg IV for 10 doses with therapeutic AUC target of 900-1500 mcmol/L (min). All patients received T-cell replete grafts. Categorical and continuous variables were compared using Pearson's chi-squared and Wilcoxon/Kruskal-Wallis, respectively. Survival estimates were calculated using the Kaplan-Meier method and curves were compared using the log-rank test. Cumulative incidence was computed as competing events using Grey's model. Univariate and multivariate analyses were performed using Cox regression modeling.
Results:
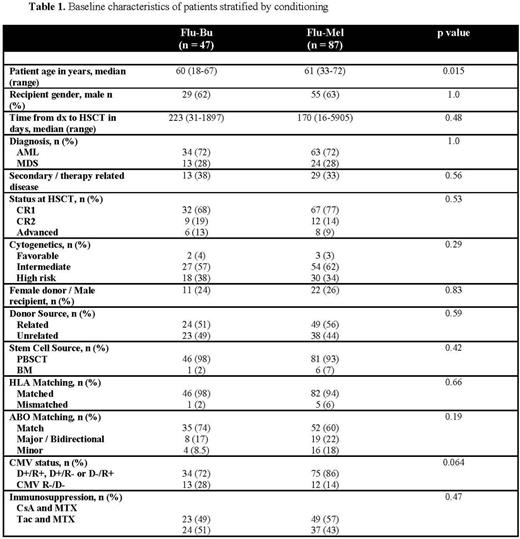
A. Baseline characteristics and AUC monitoring: 134 pts were identified (47 FB and 87 FM). Median follow up of the entire cohort was 40 months (0-63.3) and at last follow up, 60% and 29% have died or relapsed, respectively. Baseline characteristics stratified according to conditioning regimen are shown in table 1. Younger age (60 yrs FB vs. 61 yrs FM, p = 0.015) and a trend towards a higher CMV R-/D- status (28% FB vs. 14% FM p = 0.064) were the only significant differences identified. All patients in the FB group received intravenous busulfan and 46/47 patients had evaluable AUC data with a range of 732-1354 mcmol/L (min). A total of 19 patients were outside of range, all <900 mcmol/L (min) and required a median dose increase of 28.1% (3.3-50.2%)
B. Engraftment and toxicity data: The median time for platelet engraftment was 19 days (0-48) for FB vs. 16 days (14-183) for FM (p = 0.0023) whereas the median time to absolute neutrophil count (ANC) engraftment was 18 days (7-30) for FB and 15 days (11-40) for FM (p = 0.077). Cumulative incidence of grade II-IV, III-IV acute GVHD and chronic GVHD at 2-yr was 57.3%, 11% and 52.8% for FB and 48.6%, 17% and 63.4% for FM (p = 0.73, 0.4 and 0.21, respectively). Two patients in the FM group died of cardiac causes (heart failure and sudden cardiac arrest). No cases of sinusoidal obstructive syndrome were observed in either arm
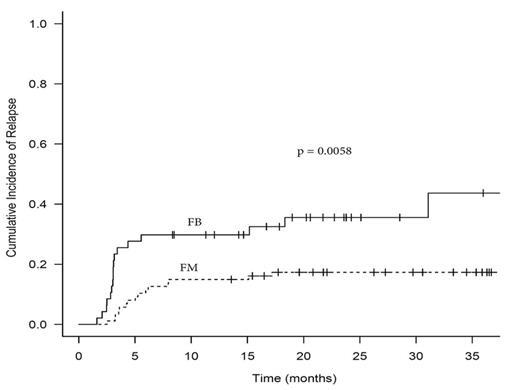
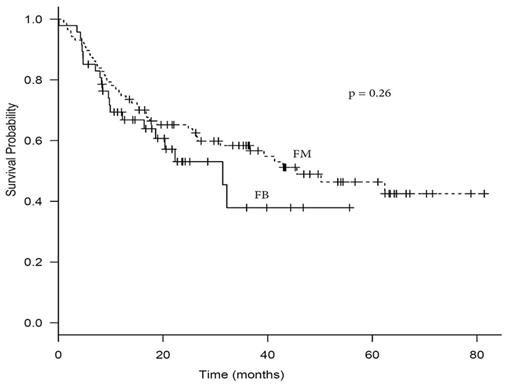
C. Transplant outcomes: A significantly higher 2-yr relapse incidence (RI) was associated with FB vs. FM at 35.6% vs 17.3%, respectively (p = 0.0058). 2-yr progression free survival (PFS) was also significantly lower in the FB vs. FM at 51.2% vs. 65.1%, respectively (p 0.031). However, 2-yr OS and NRM was similar for FB vs. FM (53.1% and 22.9% vs 63.9% and 21.9%, respectively p = 0.26 and 0.89). Necessitating a dose adjustment based on AUC did not increase the risk for relapse or affect NRM. In multivariate analysis, FB was associated with increased RI with hazard ratio (HR) 2.29 (1.07-4.88; p = 0.033). Other factors significantly associated with RI on multivariate analysis were secondary/therapy related disease with HR 2.84 (1.34-6.02; p = 0.0067) and CR1 vs. other with HR 0.39 (0.17-5.32; p = 0.019). The significance of the results remained unchanged after exclusion of MDS patients (data not shown)
Conclusion: Despite AUC dose adjustment, FB compared to FM was associated with increased RI with a similar OS and NRM. AUC dose adjustment did not impact transplant outcomes and its routine use in RIC should be further evaluated. Given the wide use of FB as a conditioning regimen, these important observations should be prospectively studied in a randomized fashion.
Al-Kali:Novartis: Research Funding. Wolf:Janssen Scientific Affairs, LLC: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal