Abstract
Introduction: The overall outcome of patients (pts) with chronic lymphocytic leukemia (CLL) who relapse after or become refractory to treatment with B-cell receptor (BCR) signaling antagonists, including ibrutinib (IBR) or idelalisib (IDE), is recently being appreciated and appears quite poor. To date, no phase 2 studies have reported efficacy in this population. Venetoclax is a selective, potent, orally bioavailable BCL-2 inhibitor with a BCR-independent mechanism of action and substantial activity in pts with heavily pretreated relapsed or refractory CLL. We report preliminary results from an ongoing phase 2, open-label study evaluating venetoclax monotherapy in CLL pts relapsed after or refractory to IBR or IDE (NCT02141282).
Methods: Pts with CLL relapsed after or refractory to IBR (Arm A) or IDE (Arm B) receive venetoclax monotherapy starting at 20 mg followed by a 5-step weekly ramp-up to a final daily dose of 400 mg. Pts with Richter's transformation (RT) suspected by screening PET CT or confirmed by lymph node biopsy are ineligible. The primary objectives are to assess the efficacy (investigator assessed overall response rate, ORR) and safety of venetoclax. Disease and response assessment was performed using iwCLL criteria at weeks 8, 24 and every 12 weeks thereafter. Adverse events (AEs) are monitored throughout the study.
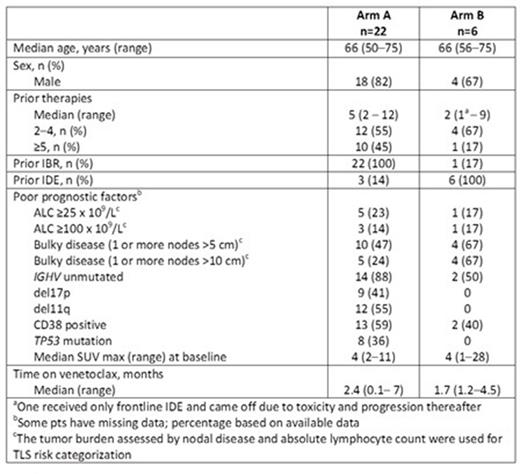
Results: As of April 30, 2015, 28 pts were enrolled in the study. Three screened pts were ineligible due to RT. Pt demographics are summarized by treatment arm in the table. Twenty-two entered into Arm A after a median duration on IBR of 15.5 months (range: 1-56). Investigator-reported best responses while on IBR prior to starting venetoclax were 14 partial response (PR), 4 stable disease (SD) and 3 progressive disease (PD); best response for 1 pt is unknown. Six entered into Arm B after a median duration on IDE of 9.7 months (range: 1-34). Investigator-reported best responses while on IDE prior to starting venetoclax were 1 complete response (CR), 3 PR and 2 SD.
At last follow-up, the median time on venetoclax was 2.4 months (range: 0.1- 7) for Arm A and 1.7 months (range: 1.2-4.5) for Arm B. Venetoclax discontinuation occurred in 4 pts in Arm A (1 each due to respiratory failure, multi-organ failure, PD of RT, death of unknown cause) and in 1 pt in Arm B (PD prior to first assessment). Fifteen pts in Arm A and 3 in Arm B underwent Week 8 response assessment. In Arm A, 8/15 (53%) achieved a PR, 6/15 (40%) had SD, and 1/15 was inevaluable. In Arm B, 2/4 achieved a PR, 1/4 had SD, and 1/4 had PD prior to first assessment. Pts with SD had evidence of ongoing disease reduction, measured by decreasing circulating lymphocytes and lymph nodes. As of the cutoff date, 23 pts remain on venetoclax therapy.
Compared to prior venetoclax monotherapy studies, no new safety signals for venetoclax were observed in either treatment arm. Treatment-emergent AEs (all grades) in >25% of the overall population were neutropenia (57%), anemia (35%), diarrhea (32%) and nausea (32%). Treatment-emergent grade 3/4 AEs in >10% of the overall population were neutropenia (43%; 3/12 of the neutropenic pts developed febrile neutropenia), anemia (29%), thrombocytopenia (18%), hypophosphatemia, hypoxia, leukopenia, and pneumonia (each 11%). Serious AEs in ≥2 pts overall were febrile neutropenia, increased blood potassium, multi-organ failure, and pneumonia (each 7%). Prior to study entry, 7/22 (32%) in Arm A received G-CSF support. One pt with high disease burden developed laboratory TLS in week 4, upon escalating to the 200 mg daily venetoclax dose, evident by hyperuricemia and hyperphosphatemia. Electrolytes returned to normal levels after a dose interruption and intervention. No pts experienced clinical TLS; laboratory changes were not clinically significant.
Conclusions: In this group of pts with aggressive disease relapsed after or refractory to BCR-targeted agents, venetoclax monotherapy demonstrated early activity at the 8 week assessment, which occurred within 3 weeks of reaching the target 400 mg daily dose. The majority of evaluable pts achieved PR or SD. Venetoclax monotherapy exhibited a tolerable safety profile without events of clinical TLS. This is the first phase 2 study to show activity in a relatively uniform population of pts previously treated with BCR kinase inhibitors; the data suggests that venetoclax is active in these pts. Enrollment in both arms was ongoing as of the data cut.
Jones:Genentech, Pharmacyclics; institutional research funding from Abbvie, Pharmacyclics, Genentech, and Gilead: Other: Advisory Board. Off Label Use: Venetoclax is an investigational drug that is not yet approved in this indication.. Mato:AbbVie: Consultancy, Research Funding; Genentech: Consultancy; Pharmacyclics: Consultancy, Research Funding; Pronai Pharmaceuticals: Research Funding; Celgene Corporation: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; TG Therapeutics: Research Funding. Coutre:AbbVie: Research Funding. Wierda:Genentech: Consultancy; AbbVie and Genentech: Research Funding. Choi:AbbVie and Gilead: Membership on an entity's Board of Directors or advisory committees; Gilead: Speakers Bureau; AbbVie: Research Funding. Davids:AbbVie and Janssen: Consultancy; Genentech and Infinity Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics, TG Therapeutics, and Infinity: Research Funding. Lamanna:Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding. Barr:Pharmacyclics: Research Funding; AbbVie and Pharmacyclics: Consultancy. Burns:AbbVie: Employment, Equity Ownership. Montalvo:AbbVie: Employment, Equity Ownership. Zhu:AbbVie: Employment, Equity Ownership. Busman:AbbVie: Employment, Equity Ownership. Potluri:AbbVie: Employment, Equity Ownership. Humerickhouse:AbbVie: Employment, Equity Ownership. Byrd:Pharmacyclics: Research Funding; Genenttech, AbbVie, Acerta, Pharmacyclics: Other: Unpaid consultant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal