Abstract

Background:
Children with severe sickle cell disease (SCD) experience organ damage, poor quality of life, and a risk of premature mortality. Allogeneic bone marrow transplantation (BMT) halts disease progression when stable donor erythropoiesis is established. Myeloablative conditioning (MAC) followed by HLA matched sibling donor BMT is effective, but most patients with SCD lack a matched sibling donor and long term side effects from MAC (e.g., infertility) can be barriers to patient acceptance of BMT. The SCURT trial was designed as a prospective, multicenter phase II trial to evaluate outcomes after HLA matched unrelated donor (MUD) BMT with reduced intensity conditioning (RIC). We hypothesized that an intensely immunosuppressive RIC regimen would be sufficient for donor engraftment and could limit toxicity. The primary endpoint was one year event free survival (EFS) (alive and disease free) ≥75%. All patients were evaluable for the primary endpoint for the purpose of this report.
Methods:
Children between 3 and 19.75 years of age who had ≥1of the following complications - stroke, high transcranial doppler velocity, recurrent acute chest syndrome, or significant vaso-occlusive pain crises were eligible. An independent review committee confirmed eligibility. The preparative regimen consisted of alemtuzumab (48 mg) (day-22 to -19), fludarabine (150 mg/m2) (day-8 to -4), and melphalan (140mg/m2) (day-3). Donors and recipients were matched at the HLA allele-level at HLA-A, -B, -C and -DRB1 loci. Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus or cyclosporine, methotrexate (7.5 mg/m2)on days +1, +3 and +6, and methylprednisolone (1 mg/kg/day) from day +7, tapered after day +28.
Results:
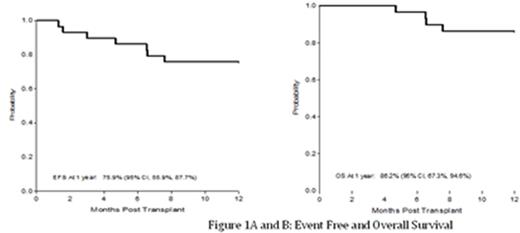
Thirty patients were enrolled between 2008 and 2014. One patient was excluded from analysis for a major eligibility violation at the DSMB's recommendation. Twenty-nine patients (16 M, 13F), 5.9-19.3 years old (median 14.0 years) from 19 centers were evaluable with median follow up of 25.2 months (range, 12-61.5 months). All patients recovered neutrophils (median 12 days) and platelets >50x109/L (median 24 days). Graft rejection (GR) in 3 patients (10%) occurred before day +100, and was followed by autologous hematopoietic recovery. No late GR has been observed. The primary endpoint was met with1-year EFS of 76% (Figure 1A). The 1-year probability of overall survival (OS) was 86% (Figure 1B). The cumulative incidence (CINC) of grade II-IV and III-IV acute GVHD at day 180 were 31% and 17% respectively (Figure 2A). The CINC of chronic GVHD at 1-year was 62%. Chronic GVHD was extensive in 38% (Figure 2B). GVHD when present was noted by 12 months post-BMT. There were 7 deaths, all in patients >14 years at time of BMT. One death occurred after a second transplant for graft rejection. The other 6 deaths occurred between 143 and 960 days post BMT, from GVHD. Non-fatal toxicities included posterior reversible encephalopathy syndrome (PRES) associated with hypertension and seizures in 35%. All cases were observed in the first 6 months post-BMT; all patients recovered with no obvious sequelae. No patient developed veno-occlusive disease, idiopathic pneumonia syndrome or cerebral hemorrhage. Infections were common, and occurred in 76% of patients. CMV and EBV reactivation each occurred in 23% of recipients. Surviving patients report performance scores of 100% (n=14), 90% (n=5), and 80% (n=3) at 2 years or at the time of last assessment. At last follow up, 13 patients had successfully discontinued immunosuppressive therapy.
Conclusion:
In summary, the RIC regimen employed in this trial of MUD BMT in children with severe SCD resulted in low rates of regimen-related organ toxicity (other than a high rate of PRES) and 1-year EFS of 76%, associated with regression of SCD manifestations. . The risk of graft rejection/disease recurrence was similar to that observed after conventional HLA-identical sibling donor BMT. Chronic GVHD occurred in 62% of patients and was the leading cause of toxicity and mortality. Future trials should focus on effective GVHD prophylaxis after URD BMT. Secondary outcome analyses including health-related quality of life and neurocognitive performance are ongoing.
Walters:ViaCord and AllCells, Inc: Other: Medical director. Levine:Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal