Abstract
Introduction
Uruguayan population is 3,4 million, mostly caucasian (88%). Life expectancy is 77 years. Cancer is the second cause of death and the estimated incidence of Multiple Myeloma (MM) is 120 cases/year. Its diagnosis and monitoring are standardized and feasible, available nationwide. Treatment with Bortezomib, Thalidomide and stem cell transplant are available for all patients, regardless of their health care provider. However, there is no accurate data on MM incidence, care, treatment-results or mortality. The Uruguayan National Myeloma Registry is designed to document current clinical characteristics of newly diagnosed MM, management and outcome in a real-world setting. This information will be useful to plan strategies to improve our local approach and follow-up of this disease, reducing problems derived from extrapolating data from other realities.
Methods
This prospective national registry will include all MM diagnosed between January 2012 and January 2015 in all institutions, public and private, nationwide. Smoldering MM are not included. Data collection started in October 2014 and is being obtained from medical and day hospital reports at each institution. Our database includes detailed data of clinical characteristics, laboratory, citogenetics and FISH, treatment indicated, disease-related and treatment-related adverse events, response to treatment at onset and relapses, and cause of death.
Results
Up to now, 163 patients have been included in the registry (45% coverage). Median age at diagnosis was 67 years (range 33-94 years), 43% younger than 66 years. Distribution according Ig subtype was: IgG 52%, IgA 27,5%, Light chains 17%, non-secretor 2,6% and IgM <1%. At diagnosis, most patients had advanced disease: 82% Durie-Salmon III, 49% ISS 3. Median bone marrow plasmacytosis was 33% and median serum monoclonal protein level was 2,14 g/dl (range 0 - 10,7 g/dl).
Anemia (hemoglobin <10 g/dl) was detected in 67% of the patients; 70% had osteolytic lesions whereas impaired kidney function (serum creatinine > 2 mg/dl) was observed in 38% and hypercalcemia in 19,7% at the time of diagnosis.
Conventional cytogenetic was performed in 66% (n=108) being normal in 78% and high-risk in 5,5%. Fluorescence in situ hybridization (FISH) for del17p, t(4;14) and t(14,16) was evaluated in 66%, being negative in 76% and positive in 24%.
The characteristics of the patients are described in Table 1.
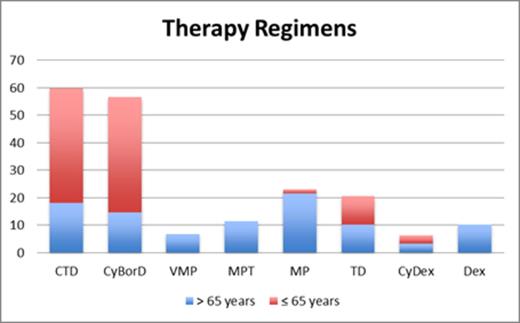
First-line treatment included at least one of the new drugs in 94% of patients younger than 65 years and in 62% of those older than 65 years. Treatment regimens are shown in Figure 1.
First-line response was available in 54% (n=88). Response rate (≥ PR) was 78%, VGPR+CR=51% and CR=19 %. Ten patients had stable disease at the end of treatment and 5 (5,7%) were relapsed-refractory MM. In ≤ 65 years, VGPR + CR was higher than in > 65 years (56% vs 33%).
Suspension or dose reductions due to treatment-related toxicity were required more frequently in patients > 65 years (50% vs 20%). Neuropathy was the most common adverse effect reported (14%).
Overall, 26% of patients received autologous stem cell transplantation (ASCT) after first line therapy; 46% in ≤ 65 years and 9% in > 65 years.
After a median follow-up of 19 months, progression free survival (PFS) was 88,6% and overall survival (OS) 78,8% (82,9% in ≤ 65 years and 78,8% > 65 years).
Discussion
This first MM National Registry provides a thorough insight into the characteristics of MM patients in our country, which may become a useful instrument to improve MM care. With a 45% coverage, we show that MM is detected in advanced stage, with a high percentage of patients with impaired renal funcion. ASCT is indicated in a low percentage, particularly in those younger than 65 years; reasons for this should be addressed in future research. Longer follow-up is needed to address the impact of new drugs in survival. To the best of our knowledge there is no other MM registry of this kind ongoing in our region. This could become a suitable platform to share with other countries in order to perform high-quality population-based research on the field.
MM clinical characteristics at diagnosis
| . | ≤ 65 years (N=70) . | >65 years (N=93) . |
|---|---|---|
| Sex: F/M (%) | 44/56 | 45/55 |
| Age (median) | 56 | 73 |
| Creatinin >2 mg/dl (%) | 44,9 | 33 |
| Hemoglobin<10g/dl (%) | 59,4 | 66,7 |
| Calcium ≥11,5mg/dl (%) | 13 | 9,7 |
| Osteolytic lesions (%) | 78 | 58 |
| . | ≤ 65 years (N=70) . | >65 years (N=93) . |
|---|---|---|
| Sex: F/M (%) | 44/56 | 45/55 |
| Age (median) | 56 | 73 |
| Creatinin >2 mg/dl (%) | 44,9 | 33 |
| Hemoglobin<10g/dl (%) | 59,4 | 66,7 |
| Calcium ≥11,5mg/dl (%) | 13 | 9,7 |
| Osteolytic lesions (%) | 78 | 58 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal