Abstract
Introduction
The prognosis of patients with multiple myeloma (MM) has improved in the last years due to the important advances in the knowledge of the biology of the disease, the implementation of new drugs and the incorporation of autologous hematopoietic stem cell transplant (autoHSCT). The allogenic hematopoietic stem cell transplant (alloHSCT) continues to be controversial: it offers a curative potential but with the cost of high toxicity, limiting the procedure to those young patients with a high-risk disease. This procedure shall be performed in expert centers and, whenever possible, in the context of a clinical trial. In the following we describe the experience of our center with alloHSCT in advance multiple myeloma patients.
Patients and methods
A total of 18 patients were diagnosed with multiple myeloma received an alloHSCT during a 13 year period (1996-2013), with a median age of 46 ± 5.9 years. All of our patients received an allogenic HLA matched sibling donor with reduced-intensity conditioning. The majority of patients were transplanted because of advanced disease, relapse after an autologous transplant or as part of a sequential transplant in patient with a high risk disease. One patient received, in two occasions, an alloHSCT. Around 70% of patients had received more than 3 previous lines of treatment including, in nearly 95%, an autoHSCT. Patient's characteristics can be found on table 1, characteristics of the procedure can be found in table 2.
| Patient«s Characteristics . | N (%) . |
|---|---|
| Gender Male Female | 10 (55,5%) 9 (44,4%) |
| Secreted Protein IgGκ IgG λ IgA κ BJ Plasmocitoma | 8 (44,4%) 4 (22,2%) 2 (11,1%) 3 (16,7%) 1 (5,6%) |
| Debut DS stage II-A II-B III-A III-B Plasmocitoma | 5 (27,8%) 1 (5,6%) 8 (44,4%) 3 (16,7%) 1 (5,6%) |
| Cytogentics at diagnosis Missing Unfavorable Favorable | 10 (55,5%) 6 (33,3%) 2 (11,1%) |
| Previous lines of treatment ²2 3-4 ³5 | 6 (33,3%) 10 (55,5%) 2 (11,1%) |
| Previous autoHSCT Yes No | 17 (94,5%) 1 (5,6%) |
| Previous radiotherapy Yes No | 8 (44,4%) 10 (55,6%) |
| Disease status at transplant Complete remission Partial remission Relapse | 9 (50,0%) 3 (16,7%) 6 (33,3%) |
| Patient«s Characteristics . | N (%) . |
|---|---|
| Gender Male Female | 10 (55,5%) 9 (44,4%) |
| Secreted Protein IgGκ IgG λ IgA κ BJ Plasmocitoma | 8 (44,4%) 4 (22,2%) 2 (11,1%) 3 (16,7%) 1 (5,6%) |
| Debut DS stage II-A II-B III-A III-B Plasmocitoma | 5 (27,8%) 1 (5,6%) 8 (44,4%) 3 (16,7%) 1 (5,6%) |
| Cytogentics at diagnosis Missing Unfavorable Favorable | 10 (55,5%) 6 (33,3%) 2 (11,1%) |
| Previous lines of treatment ²2 3-4 ³5 | 6 (33,3%) 10 (55,5%) 2 (11,1%) |
| Previous autoHSCT Yes No | 17 (94,5%) 1 (5,6%) |
| Previous radiotherapy Yes No | 8 (44,4%) 10 (55,6%) |
| Disease status at transplant Complete remission Partial remission Relapse | 9 (50,0%) 3 (16,7%) 6 (33,3%) |
| Treatment characteristics . | N (%) . |
|---|---|
| Conditioning regimen Myeloablative Reduced-intensity | 6 (33,3%) 12 (66.7%) |
| Stem cell source Bone marrow Peripheral blood | 4 (22.2%) 14 (77.8%) |
| GVHD prophylaxis CsA+MTX CsA+CS CsA+MMF | 10 (55.6%) 3 (16.7%) 5 (27.8%) |
| Infections Yes No | 16 (88.9%) 2 (11.1%) |
| Mucositis Yes No | 12 (66.7%) 6 (33.3%) |
| Acute GVHD Yes II-IV III-IV No | 4 (22.3%) 3 (16.7%) 1 (5.6%) 14 (77.8%) |
| Chronic GVHD No Limited Extensive | 8 (44.3%) 5 (27.8%) 5 (27.8%) |
| Treatment characteristics . | N (%) . |
|---|---|
| Conditioning regimen Myeloablative Reduced-intensity | 6 (33,3%) 12 (66.7%) |
| Stem cell source Bone marrow Peripheral blood | 4 (22.2%) 14 (77.8%) |
| GVHD prophylaxis CsA+MTX CsA+CS CsA+MMF | 10 (55.6%) 3 (16.7%) 5 (27.8%) |
| Infections Yes No | 16 (88.9%) 2 (11.1%) |
| Mucositis Yes No | 12 (66.7%) 6 (33.3%) |
| Acute GVHD Yes II-IV III-IV No | 4 (22.3%) 3 (16.7%) 1 (5.6%) 14 (77.8%) |
| Chronic GVHD No Limited Extensive | 8 (44.3%) 5 (27.8%) 5 (27.8%) |
Results:
Transplant related mortality (TRM) before day 100th was one case due to a thromboembolic event. Global TRM was 16.6% (3 cases). The incidence of acute graft versus host disease (aGVHD) was 22%, controlled on most cases when corticosteroids were initiated. More than half of the patients developed chronic graft versus host disease (cGVHD), with an equal distribution on either presentation as limited or extensive. (Table 2)
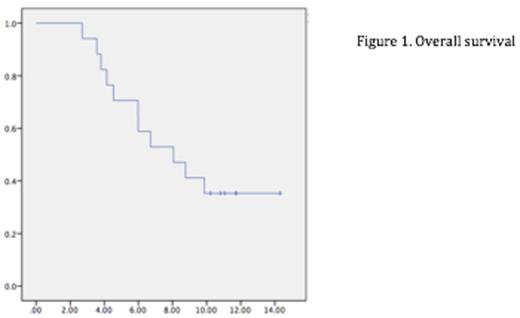
The total number of patients eligible for analysis was 17 (one patient was lost on follow-up). With a median follow up of 11 years, the overall survival (OS) was of 8.06 years [IC 95% 4,33-11,78] (figure 1.) and the estimated progression free survival (PFS) was of 25.83 months [IC 95% 8.87-42.79](figure 2). A total of 5 (29,4%) patients are still alive and 2 (11,7%) of them are in complete remission, of these 1 patient did not have a previous autoHSCT with a follow up of almost 15 years.
Conclusions: Our results are similar to those reflected on the literature1-2. However we have to point out that our population is homogenous with advanced MM with more than 3 previous lines of treatment including in most cases auto-HSCT. In spite of this, morbility and mortality in our cohort was acceptable with the limitation of a high rate of cGVHD. There is a need of more studies including more patients to evaluate the role of alloHSCT in the era of new drugs for MM.
References
1. Rosi-ol L et al. Allogeneic hematopoietic SCT in multiple myeloma: long-term results from a single institution. Bone Marrow Transplant. 2015.
2. Beaussant Y et al. Hematopoietic Stem Cell Transplantation in Multiple Myeloma: A Retrospective Study of the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Biol Blood Marrow Transplant. 2015
Alegre:Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal