Abstract
Background: In utero hematopoietic cell transplantation (IUHCT) results in long-term, multilineage chimerism across immune barriers without myeloablation/immunosuppression. It has the potential to induce donor specific tolerance (DST) for postnatal nonmyeloablative cellular transplants to treat target diseases such as Sickle cell disease. Partial deletion of donor reactive host T cells and host reactive donor T cells is responsible for tolerance and the absence of GVHD following IUHCT. The remaining nondeleted donor and host specific T cells are thought to undergo anergy. The mechanisms by which anergy and peripheral tolerance are achieved following IUHCT are not completely understood. Studies in both mice and humans have demonstrated the importance of regulatory T cells in maintaining tolerance following postnatal allogeneic transplantation. Subsets of regulatory T cells include traditional regulatory T cells (Tregs; CD4+ CD25+ FoxP3+), Type 1 regulatory T cells (Tr1 cells; CD4+ CD49b+ CD223+), and LAP+ regulatory T cells (LAP+ cells; CD4+ CD25- LAP+). These T regulatory populations inhibit T cell activation and proliferation through, among other mechanisms, the secretion of immunosuppressive cytokines including IL-10. During pregnancy, there is upregulation of circulating IL-10 levels in maternal and fetal blood, and maternal antigen specific fetal Tregs are induced in peripheral lymphoid tissue. We propose that IUHCT takes advantage of these tolerogenic features of the fetus to promote the peripheral induction of regulatory T cells capable of suppressing reactive T cell clones that escape thymic deletion.
Methods: 10x106 bone marrow cells from 6-8 week old C57Bl/6 (B6) FoxP3GFP (H2Kb) mice were injected intravenously into allogeneic gestational day 14 Balb/c FoxP3GFP fetal recipients (H2Kd). Uninjected B6 FoxP3GFP and Balb/c FoxP3GFP mice served as controls. Mice were sacrificed at 2 and 4 weeks of age. Peripheral blood donor chimerism was determined by flow cytometry at the time of sacrifice. Splenocytes were analyzed for expression of CD4, H2Kb, H2Kd, CD49b, CD223, CD25, and LAP to determine the percentage of donor and host Tregs, Tr1 cells, and LAP+ cells within the CD4+ population. CD4+ splenocytes were also assessed for intracellular IL-10 expression. Statistically significant differences between chimeric and uninjected control animals were determined using a two-tailed student's t-test.
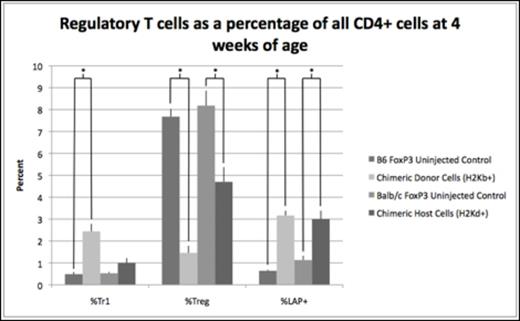
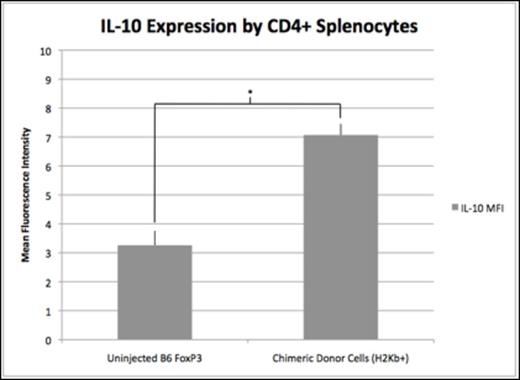
Results: Chimeric animals demonstrated a 4- and 5-fold increase in donor Tr1 cells compared to uninjected B6 FoxP3GFP controls at 2 (2.4% vs. 0.6%, p=0.03) and 4 weeks of age (2.4% vs. 0.5%, p=0.007). There was no significant difference in host Tr1 cell levels of chimeric mice compared to uninjected Balb/c FoxP3GFP controls at either 2 (0.73% vs. 0.61%, p=0.4) or 4 weeks of age (1.0% vs. 0.53%, p=0.09). Similar to Tr1 cells, LAP+ cells of donor origin were increased compared to B6 FoxP3GFP controls at 2 (2.2% vs. 0.8%, p=0.008) and 4 weeks of age (3.2% vs. 0.6%, p=0.03). Additionally, host LAP+ cells were increased compared to Balb/c FoxP3GFP controls at 2 (2.6% vs. 1.5%, p=0.0004) and 4 weeks of age (3.0% vs. 1.1%, p=0.0005). The increase in Tr1 and LAP+ cells was associated with a significant decrease in traditional Tregs in both the host and donor population at 2 and 4 weeks of age (Figure 1). Elevated levels of Tr1 and LAP+ cells did not correlate with the absolute levels of donor cell engraftment but was dependent on achieving a threshold level of chimerism known to be associated with DST. Furthermore, the MFI of intracellular IL-10 among donor CD4+ splenocytes was significantly increased compared to those from B6 FoxP3 controls at 2 weeks of age (Figure 2), demonstrating immunosuppressive activity consistent with elevated Tr1 and LAP+ levels.
Conclusion: The induction of donor and host tolerance following IUHCT is important for maintaining successful long-term engraftment and preventing GVHD. DST also allows for postnatal nonmyeloablative cellular transplants to increase engraftment to therapeutically relevant levels. We demonstrate a potentially important role that nontraditional Tr1 and LAP+ regulatory T cells play in maintaining donor cell engraftment and peripheral tolerance in the setting of IUHCT. Future investigations into the ability of these cells to augment tolerance induction after inefficient IUHCT have the potential to expand the clinical promise of IUHCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal