Abstract
BACKGROUND: Mesenchymal stem cells (MSCs) are considered to be the most promising non-hematopoietic multipotent stem cells. They are widely applied in the treatment of aplastic anemia, graft failure and GVHD post transplantation etc. However, the mechanism of MSCs homing, proliferation and the interaction with microenvironment in bone remain unclear. Based on our previous work (2014ASH poster, No.2416), a novel cell target transplantation system is developed, which is named MagIC-TT (Magnetism-induced cell target transplantation).
OBJECTIVE: To explore whether MagIC-TT can help the MSCs target homing into the bone marrow and support hematopoiesis.
Methods :
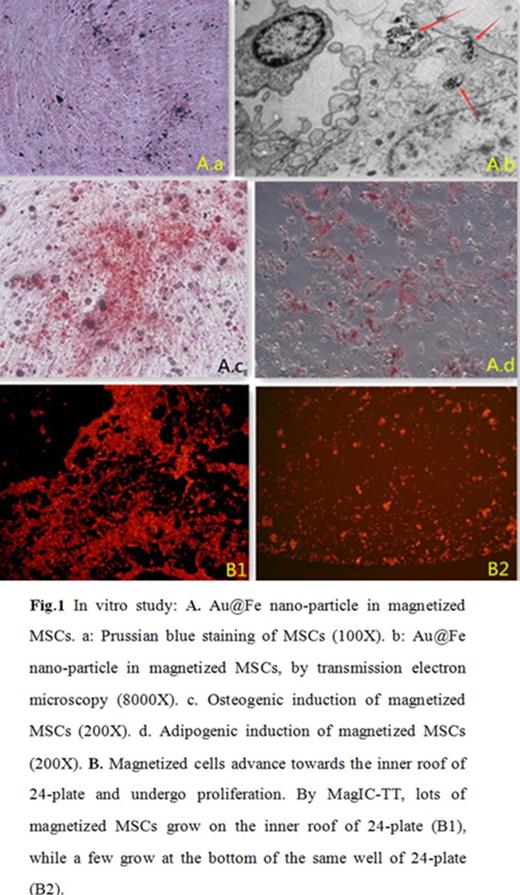
1) Magnetized cells: The C57BL/6 RFP-MSCs were magnetized by Au@Fe nano-particle, sorted by MACS column.
2) In vitro study: Both magnetized and wild type cells were compared with morphology, cell proliferation curve, cell cycle and cell differential ability. Under the effect of magnetic field, magnetized MSCs viability, migration, proliferation and co-culture with scal-1+ GFP bone marrow cells in vitro, or within 3D artificial bone scafold culture system were studied. To understand the underlining mechanism , FACS, confocal microscopy and Lightsheet Z.1 Microimage (Carl Zeiss), immunohistological staining, real-time PCR for GFP and RFP cells, deep sequencing, as well as own derived semi-solid decalcification (SSD) technique were used.
3) In vivo study: Twenty wide type C57BL/6 and another 20 female C57BL/6-Tg(CAG-EGFP) mice were used, 10 mice in each group. Magnetized MSCs were transplanted into the femur cavity of the mice by MagIC-TT, intra-femur or i.v.. Following transplantation, the above mentioned research methods as well as bioluminescence by Xenogen IVIS Image System (Lumina) were performed.
Results: 1) In-vitro study: There were no differences in cell morphology, growth curve and differentiation, etc. Prussian blue staining of MSCs showed intracytoplasmic Au@Fe nano-particle inclusions as dense blue-stained vesicles, the particles exist within or on the surface of magnetized cells (Fig.1). Magnetized cells can migrate through matrigel and transwell membranes much more efficiently under the magnetic field, 174¡À22 vs. 2¡À1 per 200X microscopic vision (P<0.0001). The cells advance toward the inner roof of 24-plate and undergo proliferation (Fig. 1). Scal-1+ GFP bone marrow cells can differentiate into megakaryocyte cells with the support of magnetized MSCs.
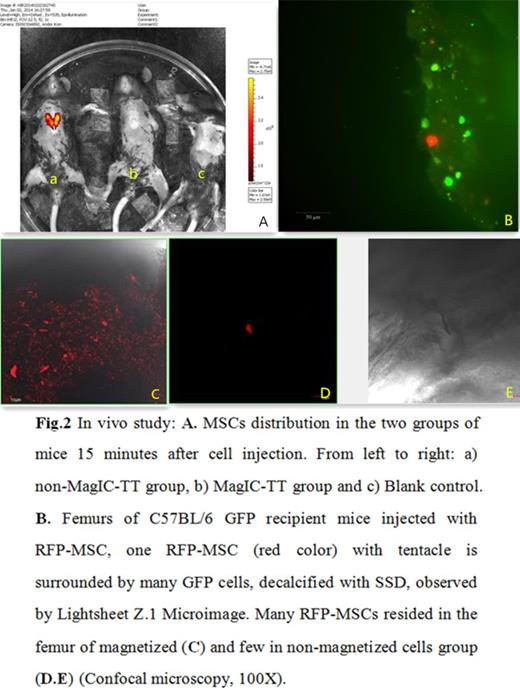
2) In vivo study: Bioluminescence assay by Xenogen IVIS Imaging System showed that MSCs were blocked in the lung in non-MagIC-TT group mice 15 minutes after cell injection, while no signal was found in the lung of MagIC-TT group mice (Fig. 2). More RFP+ cells were found in the MagIC-TT treated femur compared with the other femur in the same mouse by FACS (Table 1), pathological examination showed that lots of RFP-MSCs reside within the bone marrow MagIC-TT treated femur than the controls (Fig. 2), thereby demonstrating the success of MSCs target transplantation. The transplanted MSCs support hematopoiesis. Moreover, the relationship of RFP donor cells with surrounding cells was clearly illustrated within the bone (Fig. 2). Those MSCs were found to survive more than 3 months in the bone, lung, liver, spleen, etc.
Conclusion: MSCs target transplanted into the murine bone marrow by MagIC-TT, provide ideal support towards hematopoietic proliferation and differential ability. This technique maybe helpful to treat aplastic anemia, graft failure and GVHD, as well as other cell therapies in the future.
Comparison of the number of RFP-MSCs in two groups within 1h and 24h by flow cytometry.
| group . | 1h£¨%£© . | p . | 24h£¨%£© . | p . | ||
|---|---|---|---|---|---|---|
| *LC . | **RT . | *LC . | **RT . | |||
| BMM | 0.015¡À0.006 | 0.255¡À0.145 | 0.010 | 0.078¡À0.022 | 0.965¡À0.732 | 0.094 |
| BMW | 0.013¡À0.006 | 0.247¡À0.201 | 0.115 | 0.073¡À0.045 | 0.368¡À0.301 | 0.144 |
| P | 0.685 | 0.944 | 0.851 | 0.182 | ||
| group . | 1h£¨%£© . | p . | 24h£¨%£© . | p . | ||
|---|---|---|---|---|---|---|
| *LC . | **RT . | *LC . | **RT . | |||
| BMM | 0.015¡À0.006 | 0.255¡À0.145 | 0.010 | 0.078¡À0.022 | 0.965¡À0.732 | 0.094 |
| BMW | 0.013¡À0.006 | 0.247¡À0.201 | 0.115 | 0.073¡À0.045 | 0.368¡À0.301 | 0.144 |
| P | 0.685 | 0.944 | 0.851 | 0.182 | ||
*LC: Control femur without magnetic field (W group); **RT: Treated femur with magnetic field (M group).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal