Abstract
Introduction: Despite the enormous progress in MM therapy brought about by the rapid development of many novel agents, many patients end up with limited treatment options. We have previously reported on the efficacy and safety of metro16 in RRMM (Papanikolaou, Haematology 2013). Here we are reporting on an extension of such treatment to 28 d (metro28).
Patients and Methods: The treatment consisted of a cycle of 28d continuous iv infusions of ADR and DDP each at 1mg/m2/d, along with thalidomide 50 to 100mg/d x 28; bortezomib 0.8 to 1.0mg/m2 on days 1, 4, 7, 10, 13, 16, 19, 22, 25, 28; DEX 8 to 12mg on days 1-4, 7-10, 13-16, 19-22, 25-28; some patients also received vincristine 0.07mg flat dose by CI for 28 days. This was off-protocol therapy that patients provided written informed consent for. The IRB permitted data retrieval and analysis.
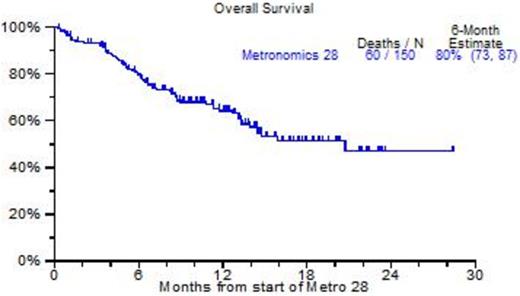
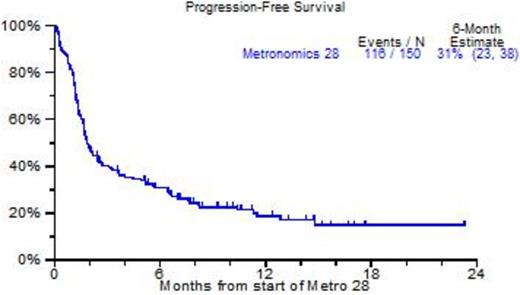
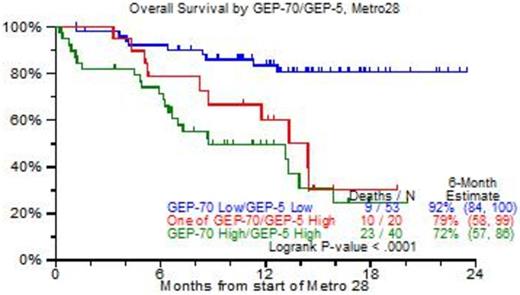
Results: 150 patients were identified, virtually all had received prior tandem transplants, bortezomib, lenalidomide, carfilzomib and pomalidomide. The median age was 64yr; B2M was elevated >=3.5mg/L in 48%, abnormal cytogenetics (CA) were present in 86%, and 44% had GEP70-defined high risk MM. Figure 1 portrays clinical outcomes. As of April 2015, 60 patients had died, and the 2-yr OS estimate was 45% (Figure 1A); the 6-mo PFS estimate was 31% although 15% had no progression at 18mo (Figure 1B). Analysis by GEP70 and GEP5 risk revealed 18-mo OS estimates of 80% among the 53 patients with low risk in both models, whereas the presence of high risk (HRMM) in either model conferred a significantly reduced 18-mo OS estimate of 25% (p<0.0001) (Figure 1C). On Cox regression analysis, OS was independently adversely affected by GEP5 HRMM (47%, HR=3.43, p<0.001), LDH >=ULN (25%, HR=3.46, p<0.001), low albumin (39%, HR=2.68, p=0.003), B2M >=3.5mg/L (51%, HR+2.63, p=0.014) and thrombocytopenia <50,000/uL (15%, HR=2.3, p=0.043). GEP5 HRMM was the sole adverse variable affecting PFS (HR=2.37, p<0.001). Most patients received metro28 in the outpatient setting, and side effects were mild.
Conclusion: Metro28 represents a well-tolerated additional tool in the treatment armamentarium for RRMM.
Clinical outcomes
A: Overall survival
B: Progression-free survival
C: Overall survival according to GEP70 and GEP5 risk designations
Clinical outcomes
A: Overall survival
B: Progression-free survival
C: Overall survival according to GEP70 and GEP5 risk designations
Schinke:University of Arkansas for Medical Sciences: Employment. Thanendrarajan:University of Arkansas for Medical Sciences: Employment. Rosenthal:Cancer Research and Biostatistics: Employment. Petty:University of Arkansas for Medical Sciences: Employment. Zangari:University of Arkansas for Medical Sciences: Employment; Onyx: Research Funding; Millennium: Research Funding; Novartis: Research Funding. Heuck:Celgene: Consultancy; Foundation Medicine: Honoraria; Janssen: Other: Advisory Board; University of Arkansas for Medical Sciences: Employment; Millenium: Other: Advisory Board. van Rhee:University of Arkansa for Medical Sciences: Employment. Epstein:University of Arkansas for Medical Sciences: Employment. Yaccoby:University of Arkansas for Medical Sciences: Employment. Morgan:Weismann Institute: Honoraria; CancerNet: Honoraria; MMRF: Honoraria; University of Arkansas for Medical Sciences: Employment; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Davies:University of Arkansas for Medical Sciences: Employment; Celgene: Consultancy; Janssen: Consultancy; Millenium: Consultancy; Onyx: Consultancy. Barlogie:University of Arkansas for Medical Sciences: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal