Abstract
BACKGROUND. In recent years, new classes of drugs have been introduced for the treatment of Multiple myeloma (MM). Several randomized trials have investigated, in the setting of first line treatment of patients eligible for autologous transplant (ASCT), the efficacy of the combination of two to four new and old drugs given as induction. Among these, VTD combination (bortezomib plus thalidomide plus dexamethasone) provided impressive results, so that it is now considered at different Institutions the standard of care as pretransplant therapy. However, data of VTD derive from clinical trials and should be verified in the "real life" setting, in terms of either efficacy or toxicity.
AIMS. Here we present our experience in 76 MM patients treated between 2008 and 2014 with VTD as part of a first line treatment including also mobilization, single or double ASCT, and consolidation with two additional VD courses. No patient had been enrolled into a clinical trial.
CHARACTERISTICS OF PATIENTS/METHODS. There were 39 males and 37 females, with a median age of 57 years (range 38-67). MM subtype was IgG=44 cases, IgA=15, IgD=1, micromolecular=13, non secreting=2, and solitary plasmocytoma=1. Stage according to Durie & Salmon was II-A=29, II-B=2, III-A=34 and III-B=10. In 8 cases single or multiple vertebroplasty was also necessary, while 7 patients had concomitant extramedullary plasmocytoma. In absence of CRAB criteria, patients were treated when progressive increase of M-component was observed. Treatment was given according to GIMEMA-MM-BO2005 protocol (Cavo et al, Lancet 2010) except for the following: from September 2012, bortezomib was given subcutaneously (in a total of 37 patients) and 4 instead of 3 induction cycles were given; mobilization therapy consisted of vinorelbine 30mg/sqm day 1 plus cyclophosphamyde 1500 mg/sqm day 2 (for further details, Annunziata et al, Ann Hematol 2006); consolidation did not include thalidomide and was given only from 2011; no maintenance therapy with dexamethasone was administered.
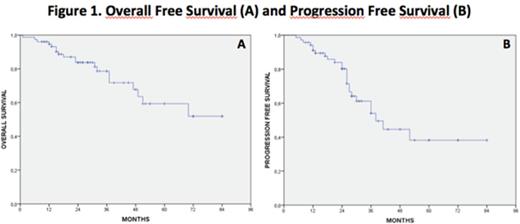
RESULTS. Overall response rate after induction was 92% (70/76 patients), with 35 complete remission (CR), 25 very good partial remission (VGPR), 9 partial remission (PR) and one minimal response (MR). One patient was considered as stable disease and continued with the therapeutic program, 3 patients were refractory and were switched to salvage therapy, and 2 patients died during induction (due to fatal sepsis from H1N1 virus infection and multiorgan failure in a severely ill subject, respectively). After successful mobilization in 70/71 patients, single (n=34) or double (n=36) ASCT were given, depending on quantity of CD34+ cell collection, toxicity of first ASCT and response achieved. High dose melphalan was the conditioning regimen in all cases. After ASCT, response was upgraded in 24 cases (in 17 cases VGPR to CR, 4 PR to VGPR, 2 PR to CR, 1 MR to PR). Consolidation was given in all 47 programmed cases. Hematologic toxicity of VTD was negligible. Reduction of thalidomide schedule was necessary in 60 patients, while only 16 patients (21%) were able to complete the programmed days of therapy at 200 mg/day. In the remaining cases, 39 completed the therapy at 100 mg, 2 at 50 mg, while 19 had to definitely discontinue therapy after a median of 33 days (15-68). More frequent reasons of discontinuation or reduction were neuropathy, constipation, fatigue and skin rash; only 1 case of thrombosis was recorded in a non responding patient. Reduction of bortezomib dose was necessary only in 5 patients (all ev cases), all because of neuropathy. At the time of writing 57/76 patients (75%) are alive, with a median follow up of 27 months. The median duration of response was 38 months, 25/70 patients (36%) having progressed or relapsed. Depending on time to relapse (> or < 18 months), bortezomib or lenalidomide based salvage therapy was used. Overall and progression free survival (OS and PFS) are shown in figure 1.
DISCUSSION. Our data demonstrate that the VTD combination in the real life is an extremely effective regimen in terms of response rate. Most patients after VTD are able to mobilize CD34+ cells as well as to receive ASCT. In a considerable proportion of cases reduction of thalidomide dose is required and in 25% of cases the drug needed to be discontinued. As compared to data from clinical trials, PFS in our series seems to be shorter, however our patients were unselected and in this series follow up is significantly longer.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal