Abstract
Introduction
Proteasome inhibitors (PI) bortezomib (B) and carfilzomib (C) are cornerstone therapies for multiple myeloma (MM). An increased incidence of PI-induced cardiac adverse events (CAEs) has been reported in patients receiving C. However, risk factors for cardiac toxicity in this population remain unclear. Our objective is to evaluate the incidence of CAEs associated with C compared with B and identify risk factors for developing events.
Patients and Method
This was a retrospective analysis of 96 consecutive patients treated for MM at Vanderbilt University from 2011 to 2014 who received B (n=44) and/or C (n=52). Patients in the C group had been previously treated with B, whereas patients in the B group did not have exposure to C. No patients studied were included in both cohorts. We evaluated the clinical features and frequency of CAEs (grade II-IV heart failure, acute coronary syndrome, left ventricular dysfunction, atrial fibrillation/flutter, thromboembolism, systemic hypertension, pulmonary hypertension, orthostatic hypotension, or sudden cardiac death). To identify factors that predisposed patients to CAEs, we analyzed duration of PI therapy, 10-year atherosclerotic cardiovascular disease (ASCVD) risk (calculated risk of myocardial infarction or stroke), gender, use of antithrombotic (antiplatelet/anticoagulant) and antihypertensive medications, prior history of cardiac events, and disease cytogenetic profile. Patients with a prior history of cardiac events were followed by a cardio-oncologist during the course of treatment.
Results
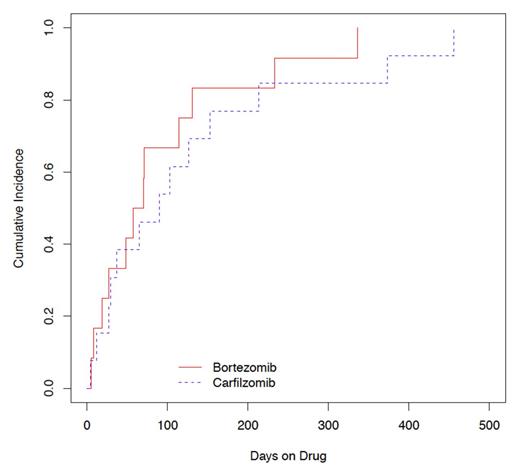
Table 1 shows patient characteristics. Twenty-five patients experienced CAEs (B, 13% (n=12); C, 25% (n=13)). Cumulative incidence (CI) of CAEs was not significantly different in patients on C compared with B (log-rank test P = 0.41) (Figure 1). Heart failure was the most common type of CAE (Table 2). CAEs occurred after a median of 90 days (range, 4-456) with C and 63.5 days (range, 5-336) with B. By univariate analysis, more patients in the C group were prior smokers, underwent stem cell transplantation and had more prior lines of therapy. More patients in the B group used antithrombotic and ACE inhibitor agents. There were no other significant differences in the use of antihypertensive, antiarrhythmic, and lipid-lowering medications between cohorts.
Multivariate analysis showed that male gender (HR 5.3, 95% CI 1.5-18.0, P = 0.007) was an independent risk factor for developing CAEs. Patients taking antithrombotic agents had a lower risk of CAE compared with those not on these therapies (HR 0.1, 95% CI 0.04-0.54, P = 0.004). While ASCVD risk was not predictive of CAEs, patients with a prior history of cardiac events who were followed by a cardio-oncologist experienced fewer CAEs (HR 0.2, 95% CI 0.05-0.72, P = 0.014). Longer duration of PI use resulted in decreasing risk of CAE (HR 0.8, 95% CI 0.7-0.9, P = 0.010). There were no interactions between these outcomes.
Conclusions
In this series, the incidence of CAEs associated with C did not differ significantly from that of B. We found that events occurred early in therapy. Male gender was an independent risk factor for CAEs. Use of antithrombotic therapy was associated with significantly reduced risk of CAEs. These data suggest that patients may benefit from antithrombotic therapy and follow-up by a cardio-oncologist while on PI therapy, particularly if there is a prior history of cardiac events.
| . | Bortezomib % (n=44) . | Carfilzomib % (n=52) . | P-value . |
|---|---|---|---|
| ASCVD Risk | 0.43 | ||
| 0-10% | 46 | 50 | |
| 10-20% | 29 | 36 | |
| >20% | 26 | 14 | |
| Male Gender | 57 | 71 | 0.82 |
| Median Age, y | 61 (38-91) | 60 (36-86) | 0.20 |
| Past Smoker | 26 | 51 | 0.02 |
| Type II Diabetes | 11 | 17 | 0.41 |
| Hyperlipidemia | 27 | 27 | 0.97 |
| Kidney Disease | 9 | 12 | 0.70 |
| Prior History of Cardiac Event | 59 | 60 | 0.96 |
| Median Duration on Bortezomib, d | 229 | 203 | 0.67 |
| Median Duration on Carfilzomib, d | 87.5 | ||
| ACE Inhibitor Use | 32 | 13 | 0.03 |
| Antithrombotic Use | 48 | 23 | 0.01 |
| ISS Stage | 0.72 | ||
| III | 34 | 25 | |
| FISH Risk | 0.13 | ||
| Standard/Intermediate | 93 | 85 | |
| High | 7 | 15 | |
| Median Prior Lines of Therapy | 0 (0-4) | 2 (0-8) | <0.001 |
| Stem Cell Transplant | 45 | 65 | 0.05 |
| . | Bortezomib % (n=44) . | Carfilzomib % (n=52) . | P-value . |
|---|---|---|---|
| ASCVD Risk | 0.43 | ||
| 0-10% | 46 | 50 | |
| 10-20% | 29 | 36 | |
| >20% | 26 | 14 | |
| Male Gender | 57 | 71 | 0.82 |
| Median Age, y | 61 (38-91) | 60 (36-86) | 0.20 |
| Past Smoker | 26 | 51 | 0.02 |
| Type II Diabetes | 11 | 17 | 0.41 |
| Hyperlipidemia | 27 | 27 | 0.97 |
| Kidney Disease | 9 | 12 | 0.70 |
| Prior History of Cardiac Event | 59 | 60 | 0.96 |
| Median Duration on Bortezomib, d | 229 | 203 | 0.67 |
| Median Duration on Carfilzomib, d | 87.5 | ||
| ACE Inhibitor Use | 32 | 13 | 0.03 |
| Antithrombotic Use | 48 | 23 | 0.01 |
| ISS Stage | 0.72 | ||
| III | 34 | 25 | |
| FISH Risk | 0.13 | ||
| Standard/Intermediate | 93 | 85 | |
| High | 7 | 15 | |
| Median Prior Lines of Therapy | 0 (0-4) | 2 (0-8) | <0.001 |
| Stem Cell Transplant | 45 | 65 | 0.05 |
Cardiac adverse events
| . | Bortezomib . | Carfilzomib . | P-value . |
|---|---|---|---|
| Total Cardiac Adverse Events* | 19 | 17 | 0.08 |
| Heart Failure | 9 | 6 | |
| Acute Coronary Syndrome | 1 | 2 | |
| Left Ventricular Dysfunction | 0 | 1 | |
| Atrial Fibrillation/Flutter | 2 | 2 | |
| Thromboembolism | 2 | 2 | |
| Systemic Hypertension | 3 | 3 | |
| Pulmonary Hypertension | 0 | 1 | |
| Orthostatic Hypotension | 2 | 0 | |
| Sudden Cardiac Death | 0 | 0 |
| . | Bortezomib . | Carfilzomib . | P-value . |
|---|---|---|---|
| Total Cardiac Adverse Events* | 19 | 17 | 0.08 |
| Heart Failure | 9 | 6 | |
| Acute Coronary Syndrome | 1 | 2 | |
| Left Ventricular Dysfunction | 0 | 1 | |
| Atrial Fibrillation/Flutter | 2 | 2 | |
| Thromboembolism | 2 | 2 | |
| Systemic Hypertension | 3 | 3 | |
| Pulmonary Hypertension | 0 | 1 | |
| Orthostatic Hypotension | 2 | 0 | |
| Sudden Cardiac Death | 0 | 0 |
*Some patients had multiple events
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal