Abstract
The aim of our perspective observational study was identify a fast molecular biomarker of tumorigenic-proliferative haematological disorders at on set using a non-invasive method. At this end, to better discriminate between myeloproliferative or lymphoproliferative hematological disease, from May to July 2014, we collected peripheral blood mononuclear cells (PBMCs) from patients at the first medical examination and without drug therapy.
The patients were divided into groups on the basis of the diagnosis. Group 2 (n=8) included patients suffered from mixed disorders such as myeloproliferative neoplasms (MPD) associated to monoclonal gammopathy of undetermined significance (MGUS). Group 3 (n=8) included patients with only MGUS, group 4 included 9 patients with only primary myelofibrosis (M).
Healthy donors (Group 1, n=16) were considered as normal subject or calibrator in molecular analysis. Their PBMCs were used to perform relativegene expression profile of a transcriptome involved in apoptotic control, stem-cell differentiation, immune network, inflammation and leukemogenesis, in order to detect whether these may serve to label the patient groups.
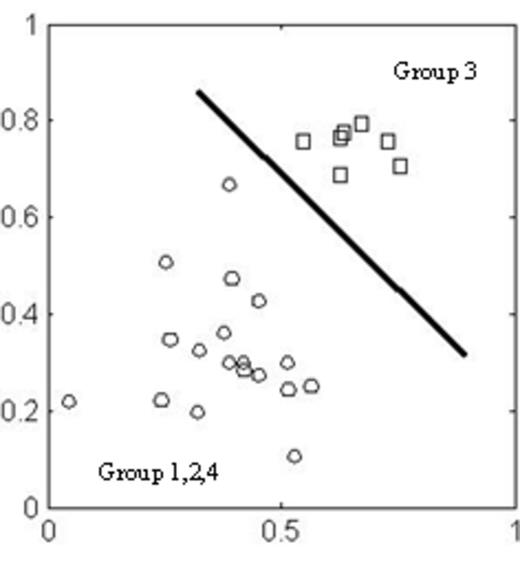
A multigene expression assay (47 genes) was carried out with the TaqM,an® Low Density Array Fluidic card. In Group 3 (MGUS), 6 genes were differentially expressed (BMI-1, FLT3, FZD1, FZD5, ICAM1, IMP3). Also, we compared variance, main value for each gene in each group and T-test that showed a significant differential expression pattern (p<0.01) for Group 3 with respect to other Groups for each of these 6 genes. Moreover, we attempted a complete class separation by applying the Support Vector Machine statistical learning algorithm with a linear kernel. Class separation is highlighted in Fig. 1. Group 3(MGUS) was completely separated from other groups MPD/MGUS, M and from normal subjects. In Group 3(MGUS), all 6 genes were over-expressed respect to the normal group. Of note, we were unable to identify any specific markers in myeloproliferative disorders evaluated in parallel.These results describe the altered expression of a subset of genes including BMI-1 (polycomb ring finger oncogene) in PBMCs samples from patients suffering from MGUS. In order to understand the significance of the high expression of BMI1 in MGUS-PBMC s and if it could be prognostic of malignancy, we performed comparative BMI1 relative expression in the bone marrow samples (BM) and PBMCs taken from 5 patients with multiple myeloma (MM) at first diagnosis and without drug treatment. In this case, we used BM samples as normal calibrator of 5 healthy donors volunteers (control group), too. In all MM samples, the expression of the BMI1 was significantly high than control group (p<0,001). MM group and control group were compared using the T-test. In June 2015, we considered the disease evolution in MGUS patients evaluated as first diagnosis in 2014..4 of eight patients showed rapid evolution of malignant disease.We propose that the perspective screening for BMI-1 expression alone or in combination with other molecular markers may help refine disease staging and prognosis towards the optimization of therapeutic interventions. Our data indicate that the evaluation of the BMI1gene expression in total PBMCs may represent a more practical diagnostic test using more easily obtainable and less expensive biologic material, thus avoiding more invasive procedures in patients.
We applied a SVM classification simulation with a linear kernel regression on a sub dataset with the six genes identified. It is easy to make a good class separation for Group 3( MGUS.) vs other groups (MPD/MG) and M.
We applied a SVM classification simulation with a linear kernel regression on a sub dataset with the six genes identified. It is easy to make a good class separation for Group 3( MGUS.) vs other groups (MPD/MG) and M.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal