Abstract
Background: Myelodysplastic syndrome (MDS) is a heterogeneous disease characterized by cytopenias and a propensity to progress into acute myeloid leukemia in a subset of patients. Clinical trials which led to FDA approval of hypomethylating agents (HMAs) stratified patient's risk status per the IPSS. In 2008, WHO further categorized patients into different groups and the IPSS was revised into the IPSS-R. Therefore, there is a paucity of data regarding the response rates and clinical outcomes of HMA therapy when stratified per WHO subtype and IPSS-R.
Goal: We carried this review to assess whether WHO subtypes or IPSS-R had a differential response with corresponding impact on survival.
Methods: After IRB approval, all MDS pts who received hypomethylating agents (HMA) (azacitidine or decitabine) with available response data (2000-2014) were included. Clinical characteristics were obtained retrospectively. IPSS-R (Greenberg 2012) was used for risk stratification. Response assessment was based on IWGR 2006 criteria. Statistical comparisons were performed with the χ2 test for categorical variables and the Wilcoxon test for continuous variables. Survival analysis was based on the Kaplan-Meier method and log rank test. For all analyses, P ≤ 0.05 was considered statistically significant.
Results: We identified 823 pt in our MDS database; among those that received HMA therapy 70 patients (pts) had evaluable response data. The median age at diagnosis was 67 years (31-85) and 49 (70%) were males. MDS subtypes per WHO 2008 classification were 31(44%) RAEB1/2, 26(37%) RCMD, 10(14%) MDS-U, 2(3%) RARS, and 1(1%) MDS 5q. Fifteen (33%) patients had secondary MDS. 23(32%) developed AML, and median time to leukemic transformation was 14 months (7.8-21). Median time to start HMA was 2.7 months (0-77). Median follow up from initial diagnosis was 20 months (3-118) at which time 22(31%) deaths were documented.
A. Patient characteristics: Prior to HMA therapy median laboratory values included; Hemoglobin 9.6 g/dL (6.6-14.8), ANC 1.1 x1010 (0.01-16), Platelet 66 x1010 (4-937), peripheral blast % 0 (0-18), and bone marrow blast % 5 (0-17). IPSS-R for our cohort was as the following, 15(27%) Very high, 14(25%) high, 10(18%) intermediate 12(21%) low, and 5(9%) very low risk.
B. HMA Response rates based on WHO subtype:
We observed no significant difference in response (p = 0.6) when comparing response rate among different groups. Response rates per WHO subtype were as follows:
i. RAEB: 7 (22%) pts achieved CR/Marrow CR, 1(3%) had HI/PR, 17(55%), and 6(20%) had stable disease (SD) and progressive disease (PD), respectively.
ii. RCMD: 2 (8%) pts achieved CR/Marrow CR, 4(16%) had HI/PR, 12(46%), and 8(30%) had SD and PD, respectively.
iii. MDS-U: 1 (10%) pt achieved CR/Marrow CR, 1(10%) had HI/PR, 6(60%), and 2(20%) had SD and PD, respectively.
iv. MDS 5q/RARS: 1(33%) had SD, and the remaining three pts had PD.
C. HMA Response rates based on IPSS-R:
i. Very low: 1(20%) had HI/PR, 3(60%), and 1(20%) had SD.
ii. Low: 2(17%) pts achieved CR/Marrow CR, 3(25%) had HI/PR, 4(33%), and 3(25%) had SD and PD, respectively.
iii. Intermediate: 2 (20%) pts achieved CR/Marrow CR, 5(50%), and 3(30%) had SD and PD, respectively.
iv. High: 2(14%) pts achieved CR/Marrow CR, 2(14%) had HI/PR, 7(50%), and 3(21%) had SD and PD, respectively.
v. Very high: 1(7%) pts achieved CR/Marrow CR, 9(60%), and 5(33%) had SD and PD, respectively.
Comparing responses among IPSS-R risk stratification groups, was not statistically significant (p = 0.49). We did not observe a difference in response rate when we further classified pts into low risk (very low, low & intermediate), and high risk (high, very high) (p = 0.27).
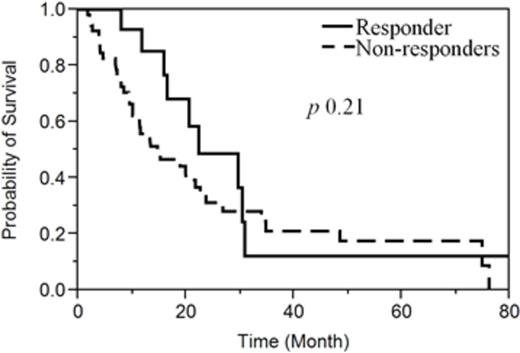
D. Response effect on Survival: Overall survival was not statistically different among responders (CR/Marrow CR/HI &PR) and non-responder (SD & PD); 22.3 vs 14.7 months respectively (p = 0.21) (Figure1).
Conclusion: Using contemporary MDS risk stratification and WHO classification was not predictive of response to hypomethylating agents. Responders did not seem to benefit from prolongation of survival. Given the small sample size in this cohort, this should be further explored in larger groups to identify if there are any potential subgroups that would benefit more from such therapy.
Al-Kali:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal