Abstract
Introduction: The incidence of invasive fungal infections (IFI) and need for antifungal prophylaxis in patients with high-risk myelodysplastic syndromes and acute myeloid leukemia (MDS/AML) treated with hypomethylating agents is currently unknown.
Methods: We retrospectively analyzed the incidence of IFI in all MDS/AML patients receiving azacitidine (75 mg/m2/day 7 days every 28-day cycle) in our center between June 2007 and June 2015. IFI diagnosis follows the European Organization for Research and Treatment of Cancer and Mycosis Study Group (EORTC/MSG) 2008 Criteria. Patients did not receive antifungal prophylaxis.
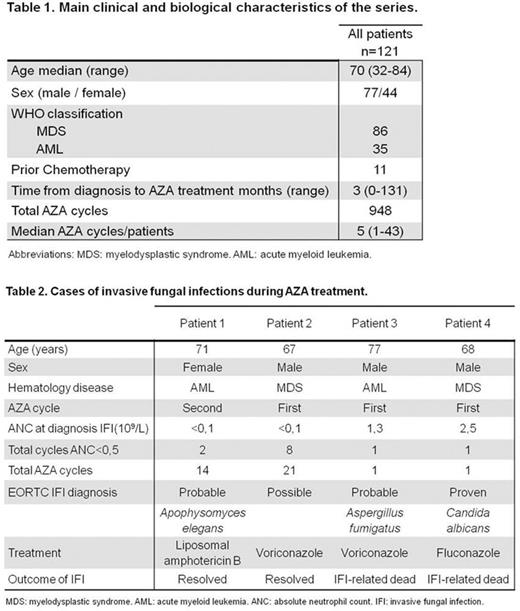
Results: One hundred and twenty one consecutive unselected patients (86 MDS and 35 AML; Table 1) received a total of 948 cycles of azacitidine in this series (median 5, range 1-43). One hundred and ten (91%) received azacitidine frontline and 11 (9%) following refractoriness or relapse after previous intensive chemotherapy. Seventy one patients received ≥4 azacitidine cycles. Forty two (35 %) patients had complete medullar and cytogenetic responses. Two hundred and seventy four azacitidine cycles (28%) in 49 patients (40%) were administered during and/or led to prolonged grade IV neutropenia (<0.5x109/L ³10 days), and caused febrile neutropenia episodes in 121 cycles (12% of total, 44% of neutropenic cycles) in 45 patients (37%), range 1-6 per patient. Four IFI occurred in these patients: one possible, two probable and one proven (Table 2). The incidence of IFI was 0.42% per treatment cycle and 3.3% per patient treated for the overall series, and 0.72% per treatment cycle and 4.1% per patient treated among those with prolonged severe neutropenia. Of note, all cases of IFI occurred in either the first or the second azacitidine courses. Two patients died from IFI, leading to an IFI-related mortality rate of 1.65% per patient treated and 0.21% per treatment cycle. The numbers needed to treat with primary prophylaxis, even for an ideal agent that could potentially prevent all IFI, are well in excess of 100 courses of azacitidine and well over 20 patients throughout their treatment course, to prevent one single IFI in these patients, even among those with neutropenia.
Conclusion: Our data show that patients with MDS/AML treated with azacitidine have a very low risk of IFI, including those with concurrent severe prolonged neutropenia. Primary antifungal prophylaxis should not be recommended for this subset of patients. This very low risk of IFI is a potential additional benefit from treatment of MDS/AML with hypomethylating agents, compared to conventional intensive chemotherapy. Prospective studies are needed to better address this issue.
Sureda:Takeda: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal