Abstract
Introduction: Erythropoiesis-stimulating agents (ESAs) have been used in treating anemic MDS patients to improve erythropoiesis and reduce the risk of red blood cell (RBC) transfusion. Two previous systematic reviews found that ESA use was efficacious, but these reviews focused on short-acting epoetin alfa (EA). Since then, the results of a number of prospective interventional trials of DA have been reported. We present an updated systematic review and meta-analysis to estimate the efficacy of DA in the treatment of MDS-related anemia.
Methods: We conducted a systematic review of the medical literature to identify prospective interventional trials of DA in patients with MDS. The main inclusion-exclusion (IE) criteria were: that studies had to be prospective and interventional in nature; have at least 10 adult subjects with MDS reporting either World Health Organization (WHO), French-American-British (FAB) criteria, or International Prognostic Scoring System (IPSS) status; and report results for the pre-specified primary outcome (proportion of patients with erythroid response) or one of the secondary outcomes (which included major hemoglobin response, changes from baseline in hemoglobin levels, transfusion status, and quality of life (QoL) measures). We recorded and collated all reported adverse events. Two independent reviewers identified the studies and abstracted the information and a third reviewer adjudicated. Clinical and methodological heterogeneity across studies were evaluated with respect to the study population and participant selection method (e.g. MDS diagnosis, history of ESA use, baseline erythropoietin (EPO), hemoglobin, and creatinine levels, transfusion status, and other factors), the intervention (e.g. initial and maintenance ESA dose), and the endpoints of interest including the response criteria. Forest plots with formal testing using Cochran's Q-statistic was also used to assess the heterogeneity across the studies. We used random effects methodology to generate combined estimates when warranted by the data. Subgroup analyses and/or meta-regressions were conducted by dose level, ESA-naïve status, baseline EPO level, hemoglobin level, transfusion status, and other factors.
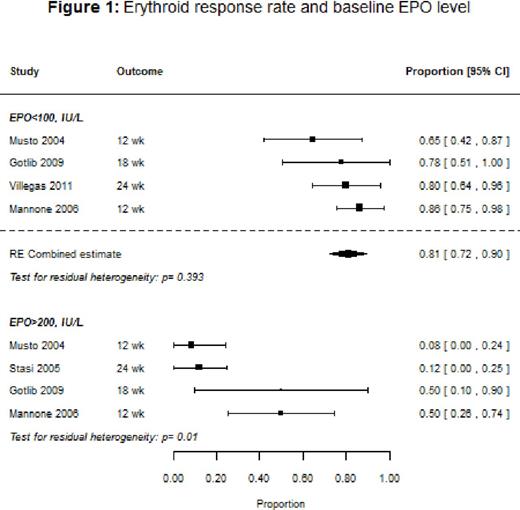
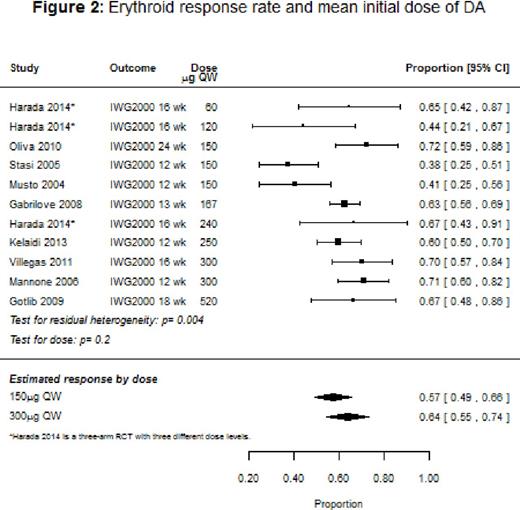
Results: Ten studies (9 single-arm, 1 randomized controlled trial [RCT]) with a total of 647 patients were included in the systematic review, with response rates ranging from 38% to 72%, and the median duration of response varying from 5 to 12 months. The overall response rate for the nine studies that used IWG 2000 response criteria could not be evaluated because of heterogeneity. However, among studies that stratified response rates by baseline endogenous EPO levels, patients with EPO <100 IU/L had an overall response of 81% (95% confidence interval [CI]: 73-90%), and on average a 39% [95% CI: 22-56%] better response than patients with EPO >100 IU/L. Across the studies that reported response stratified by prior ESA use, ESA-naïve patients had a median response rate of 75% compared to 53% for patients previously treated with an ESA. Baseline mean hemoglobin was significantly associated with response (p=0.0204), with higher baseline levels associated with improved response. We also found that response tended to improve with dose (p=0.2; Figure 2): the estimated response for a mean initial dose of 150 μg once weekly (QW) was 57% (95% CI: 49-66%) compared to 64% (95% CI: 55-74%) for 300 μg QW. Baseline transfusion independence (6 studies), and low-risk IPSS status (2 studies) were reported to be significantly associated with better response in several studies. Treatment with DA tended to show an improvement in the quality of life (QoL) measures, transfusion rates, and hemoglobin levels, but the number of studies that reported these outcomes was small. Hypertension, thromboembolism, and progression to acute myeloid leukemia were respectively reported in 2%, 1%, and 1% of the patients.
Conclusions: Published studies suggest that treatment with DA yielded high hemoglobin response (38-72%) in MDS patients with anemia. The response was strongest in patients with lower baseline serum EPO level, and ESA naïve patients.
Park:Hospira: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding. Fenaux:Amgen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Celgene Corporation: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Mehta:Amgen Inc.: Employment; Amgen Inc.: Equity Ownership. Callaghan:Amgen Inc.: Employment; Amgen Inc.: Equity Ownership. Kim:Amgen Inc.: Employment; Amgen Inc.: Equity Ownership. Tomita:Amgen Inc.: Employment; Amgen Inc.: Equity Ownership. Xu:Amgen Inc.: Employment; Amgen Inc.: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal