Abstract
Introduction
Individuals with the Philadelphia chromosome negative myeloproliferative neoplasms (MPNs) have previously been shown to experience symptoms in excess to age and comorbidity matched peers. Although numerous assessments of symptom burden and quality of life have been utilized among MPN populations, including the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30), Patient Reported Outcomes Measurement Information System (PROMIS¨) fatigue scale, and Functional Assessment of Cancer Therapy-Lymphoma (FACT-Lym) and the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-10), there are currently no validated assessments of overall health. Developed by the EuroQol Group to assess heath status, the EQ-5D is an internationally validated self-assessment which may be useful in the assessment of health state among MPN patients (Annals of Medicine, 2001. 33:337-43).
Methods
The EQ-5D evaluates five dimensions of mobility, self-care, usual activities, pain/discomfort and anxiety/depression, on a scale from no problems to extreme problems and assesses patient reported health state via a numerical visual analogue scale (VAS) ranging from 0 to 100. The health state score is a composite score of these items ranging from 0 to 1.0, with higher scores indicating an improved health state. EQ-5D survey participants were essential thrombocythemia (ET) and polycythemia (PV) patients participating in the MAJIC United Kingdom (UK) trial. The MAJIC trial is a randomized, phase II study of best available therapy versus JAK inhibition in individuals with PV or ET who have been resistant or intolerant of cytoreduction with hydroxyurea /hydroxycarbamide. This study was one of the first Trials Acceleration Programme (TAP) trials and is funded by Leukaemia Lymphoma Research, with drug supplied by Novartis. Only baseline EQ-5D assessments taken at study enrollment were included in this analysis. Comparative analysis was preformed using the MPN-10 (J Clin Oncol.2012. 30:4098-103).
Results:
Participant Demographics: Sixty six patients with ET and 55 patients with PV completed the EQ-5D questionnaire. Median respondent age was 65 years old and half of respondents were female (54%). Average MPN duration was 10 years for ET and 9.2 years for PV.
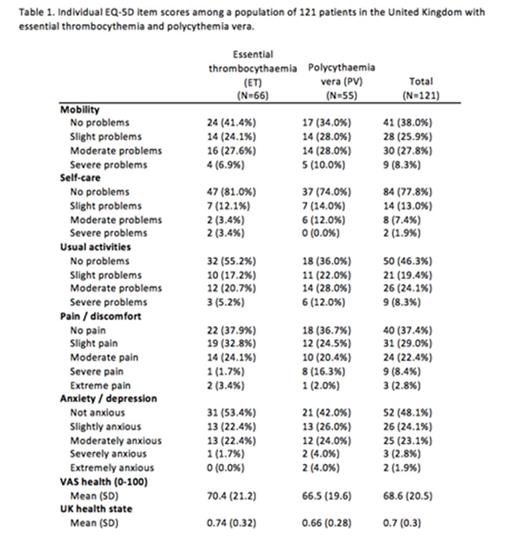
EQ-5D Scoring: Severe to extreme difficulties in the items of mobility, self-care, usual activities, pain/discomfort and anxiety/depression were common (Table 1).Patients with PV tended to have higher proportion of patients with severe to extreme difficulties in individual disease dimensions, higher VAS score, and worsened health state compared to their essential thrombocythemia counterparts, although these effects were not significant. Average VAS health score was 70.4 (+21.2) for ET and 66.5 (+19.6). When evaluating the health state composite score, the mean ET score was 0.74 (+0.32) and mean PV score was 0.66 (+0.28).
EQ-5D Validation: The EQ-5D had excellent internal consistency (CronbachÕs alpha=0.87) and strong internal correlation. When evaluating external validity, strong correlations existed between the EQ-5D and the MPN-SAF TSS scales for dimensions of mobility, usual activities, pain and overall health state (many items with r >0.5 and p<0.001).
Conclusions:
The EQ-5D is a valid and accurate instrument for the brief assessment of health state among MPN patients. This instrument demonstrates excellent clinical utility in a large clinical study cohort and can be used in both clinical practice and trial settings. When comparing EQ-5D health state scores, ET and PV patients had worsened health than the general UK population age 60 to 69 (0.774; Med Decis Making. 2011.31(6)800-4), but similar health to individuals with other malignancies including breast cancer (0.760), prostate cancer (0.743), and lung cancer (0.689).
Mesa:NS Pharma: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Incyte Corporation: Research Funding; Pfizer: Research Funding; Promedior: Research Funding; CTI Biopharma: Research Funding; Gilead: Research Funding; Genentech: Research Funding; Pfizer: Research Funding. Harrison:CTI Biopharma: Consultancy, Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Shire: Speakers Bureau; Gilead: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal