Abstract
Background: The development of tyrosine kinase inhibitors (TKIs) has dramatically changed the landscape of chronic myelogenous leukemia (CML) treatment. Dosing of TKIs is standardized and is based on the phase of disease at presentation, but is not altered based on body surface area (BSA), sex, or other patient characteristics. These medications are generally well tolerated, however, hematologic and non-hematologic toxicities are not infrequent. While the package insert of all three TKIs (imatinib, dasatinib, nilotinib) commonly used in first line therapy provides specific dose-reduction instructions for hematologic toxicity, only nilotinib has a standard recommended dose for nonhematologic toxicity. Long-term data on the effects of dose-reduction of TKI therapy based on toxicity is lacking. Furthermore, there is little data on risk factors for TKI intolerance. Thus, we sought to identify characteristics of patients requiring dose reductions as a part of first-line TKI treatment for chronic phase CML as well to determine the long-term outcome of these patients in a retrospective fashion.
Methods: Using billing records, we identified all patients seen at our institution for treatment of chronic phase CML between November 1, 2010 and July 16, 2015. We then reviewed the records of all such patients to identify individuals requiring chronic dose reductions of first-line treatments for chronic phase CML. We identified a control group of patients by capturing all patients tolerating full dose first-line TKI therapy during a pre-specified period. This control group was used to determine risk factors for TKI intolerance.
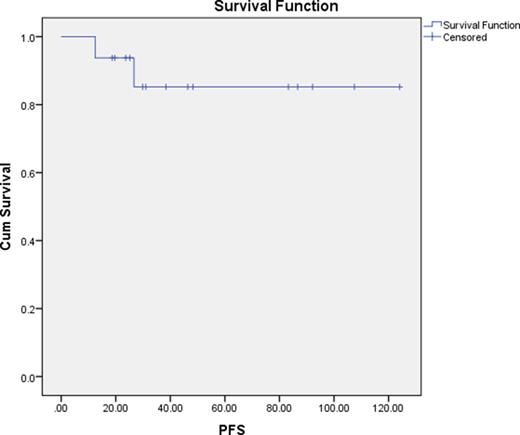
Results: A total of 18 patients were identified who required long-term dose reduction of first-line TKI therapy with a median follow up time of 34.72 months (range 12.47-124.07). The mean time on full dose therapy prior to dose reduction was 7.08 months (range 0-37.27). The median time spent on reduced dose therapy was 26.37 months (range 4.90-118.93). Eighty-nine percent of patients remained in a major molecular response 4 (MR4) despite dose reduction. Median progression free survival (PFS), as defined by loss of MMR, of dose-reduced patients had not yet been reached. One, two, and three year PFS rates were 100%, 93.8%, and 85.2% respectively. When compared to the control group of patients tolerating front line therapy at full-dose, patients requiring dose reduction were more likely to be female: 78% vs 48% (P=0.045) and have a lower BSA: 1.77 versus 1.99 mg/m2 (P=0.010).
Discussion: This small, retrospective analysis identifies several potential important risk factors for TKI dose reduction including female sex and lower BSA. Based on the available control group, it cannot be determined if either of these are independent variables. The majority of patients remained in MR4 despite dose reductions. Both of the 2 patients (12.5%) who did not were able to regain MR4 status after switching to an alternate TKI, suggesting that long-term treatment with reduced dose TKIs is reasonable so long as patients are closely monitored. Our data suggest the possible utility of dose adjustments based on BSA for patients who are intolerant of TKI therapy.
TKI administered degree of dose reduction.
| Patient Number . | TKI . | Dose . |
|---|---|---|
| 1 | Dasatinib | 20 mg QOD |
| 2 | Dasatinib | 10 mg daily |
| 3 | Dasatinib | 40 mg daily* |
| 4 | Imatinib | 300 mg daily |
| 5 | Imatinib | 200 mg alternating with 300 mg daily |
| 6 | Dasatinib | 40 mg daily |
| 7 | Imatinib | 300 mg daily* |
| 8 | Dasatinib | 50 mg daily |
| 9 | Dasatinib | 40 mg daily |
| 10 | Dasatinib | 70 mg daily |
| 11 | Nilotinib | 450 mg daily |
| 12 | Dasatinib | 40 mg daily |
| 13 | Nilotinib | 450 mg daily |
| 14 | Dasatinib | 50 mg daily |
| 15 | Dasatinib | 40 mg daily |
| 16 | Imatinib | 300 mg daily |
| 17 | Dasatinib | 35 mg daily |
| 18 | Dasatinib | 70 mg daily |
| Patient Number . | TKI . | Dose . |
|---|---|---|
| 1 | Dasatinib | 20 mg QOD |
| 2 | Dasatinib | 10 mg daily |
| 3 | Dasatinib | 40 mg daily* |
| 4 | Imatinib | 300 mg daily |
| 5 | Imatinib | 200 mg alternating with 300 mg daily |
| 6 | Dasatinib | 40 mg daily |
| 7 | Imatinib | 300 mg daily* |
| 8 | Dasatinib | 50 mg daily |
| 9 | Dasatinib | 40 mg daily |
| 10 | Dasatinib | 70 mg daily |
| 11 | Nilotinib | 450 mg daily |
| 12 | Dasatinib | 40 mg daily |
| 13 | Nilotinib | 450 mg daily |
| 14 | Dasatinib | 50 mg daily |
| 15 | Dasatinib | 40 mg daily |
| 16 | Imatinib | 300 mg daily |
| 17 | Dasatinib | 35 mg daily |
| 18 | Dasatinib | 70 mg daily |
*Indicates loss of MR4 while on therapy
Risk factors for TKI dose reduction.
| . | Full dose group . | Dose reduction group . | P value . |
|---|---|---|---|
| Number | 29 | 18 | |
| Gender Male Female | 15 14 | 4 14 | 0.045 (chi-square) |
| BSA | 1.99±0.28 | 1.77±0.25 | 0.010 (t test) |
| BMI | 30.27±6.34 | 27.20±4.28 | 0.084 (t test) |
| . | Full dose group . | Dose reduction group . | P value . |
|---|---|---|---|
| Number | 29 | 18 | |
| Gender Male Female | 15 14 | 4 14 | 0.045 (chi-square) |
| BSA | 1.99±0.28 | 1.77±0.25 | 0.010 (t test) |
| BMI | 30.27±6.34 | 27.20±4.28 | 0.084 (t test) |
PFS as defined by loss of MMR for patients on chronically dose-reduced TKIs.
PFS as defined by loss of MMR for patients on chronically dose-reduced TKIs.
Koprivnikar:Alexion: Consultancy. McCloskey:Novartis: Consultancy; Pfizer: Consultancy. Goldberg:Pfizer: Research Funding; COTA: Employment, Equity Ownership, Other: Leadership, Stock; Ariad: Research Funding, Speakers Bureau; BMS: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Faderl:Celgene Corp.: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal